��
mast cell activation syndrome~treated by Gleevec prevents a key mast cell element from activating, Afrin said, and in some patients halts or reduces allergic reactions
Struck by mast cell activation syndrome, Jennifer credits M Health expert with saving her life | MHealth.org
https://www.mhealth.org/blog/2015/august-2015/struck-by-mast-cell-activation-syndrome-jennifer-credits-m-health-expert-with-saving-her-life��
Mast cells are especially concentrated in small blood vessels and epithelial tissues covering the skin and lining the respiratory and digestive tracts.
Mast cells are also found in tumors, osteoathritic joints, mast cells are aberrantly activated in human and murine osteoarthritic joint tissues. mast cells are aberrantly activated in human and murine osteoarthritic joint tissues (1~3%).~a central role for IgE-dependent mast cell activation in the pathogenesis of osteoarthritis, and in rheumatoid arthritis (Lee et al., 2002) and gouty arthritis
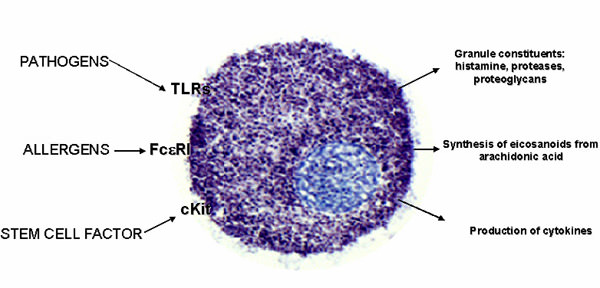
Activation of mast cells~IgE,TLRs,ocmplement,
IgE-mediated mast cell activation promotes inflammation and cartilage destruction in osteoarthritis
VA Palo Alto Health Care System, United States; Stanford University School of Medicine, United States; University of Padova, Italy; Virginia Commonwealth University School of Medicine, United States
Mast cells are sentinels of the innate immune system, poised to rapidly respond to exogenous pathogens and to endogenous danger signals (Bischoff, 2007). A wide variety of stimuli (e.g., allergens that cross-link IgE-bound high affinity IgE receptor (Fc��RI) or antibodies that directly cross-link Fc��RI, cytokines such as IL-33, complement anaphylatoxins, immune complexes, neuropeptides, TLR ligands, etc.) can influence mast cell degranulation and release of pre-formed mediators including histamine, tryptases, pro-inflammatory lipids, cytokines and chemokines (Theoharides et al., 2012; Yu et al., 2016). Importantly, different activation stimuli are capable of inducing distinct mast cell responses in both physiological and pathological settings (Enoksson et al., 2011; Gaudenzio et al., 2016). In allergic disease��a setting in which mast cells have been most extensively studied��these mediators promote chronic allergic inflammation which, if sustained, results in long-term tissue damage, fibrosis, and remodeling (Galli and Tsai, 2012). Similar to the tissue remodeling in allergic diseases, human osteoarthritis and experimental osteoarthritis in rodents are characterized by abnormal and progressive bone and other tissue remodeling (Remst et al., 2014).
IgE-mediated mast cell activation promotes inflammation and cartilage destruction in osteoarthritis | eLife
https://elifesciences.org/articles/39905��
https://nutritionreview.org/2013/04/natural-allergy-relief/
��
��
Cells | Free Full-Text | Mast Cells in Liver Fibrogenesis
https://www.mdpi.com/2073-4409/8/11/1429
��
Do mast cells hold the key to chronic and unexplained disease?
Cytology of a mast cell. Image credit: Rayya the Vet, via NIH Image Gallery.
Dr. Jennifer McClure reflects on evidence about mast cells as a key to chronic disease, providing new insights for physicians, researchers, and the public.
by Jennifer McClure, PhD, Group Health Research Institute Senior Investigator and Director of Research, Faculty & Development
https://www.kpwashingtonresearch.org/news-and-events/blog/2016/june/do-mast-cells-hold-key-chronic-and-unexplained-disease��
Natural Allergy Relief
By NutritionReview.org -April 19, 2013126300Understanding how the right combination of herbs can work to 1) gently interrupt the allergic response, 2) reduce inflammation and 3) aid in resolving the underlying imbalance requires a brief review of the three stages of the allergic response: sensitization, acute inflammation, and chronic tissue damage.
Stage 1: Sensitization to Allergens
In Stage 1, for reasons not yet fully understood, the immune system mistakenly identifies an otherwise harmless substance as a potential threat to the body. While there are no allergic symptoms at this stage, the immune system is already hard at work preparing for battle with the newly targeted allergen.
The process starts as a specialized class of cells called macrophages attach themselves to the targeted allergen. After destroying the substance, the macrophages pass fragments of the allergen on to a class of cells called T-lymphocytes (T-cells). T-cells respond by secreting acytokine, called interleukin-4, that triggers another class of lymphocytes, B-lymphocytes, to begin producing immunoglobulin E (IgE) antibodies specific to that allergen.
Individuals prone to allergies are known to create abnormally high levels of IgE antibodies, making them susceptible to allergic rhinitis, asthma, atopic dermatitis and anaphylactic reactions.
Mast Cells
As IgE antibodies circulate through the blood, they attach to receptors on mast cells located in connective tissues. Mast cells are especially concentrated in small blood vessels and epithelial tissues covering the skin and lining the respiratory and digestive tracts (Fig. 1). In addition to mast cells, IgE antibodies also attach to basophils, a specialized type of white blood cell that can exit small blood vessels to congregate around allergens. Once this first stage is complete, a future exposure will cause the targeted allergen to bind to the newly created IgE molecules residing on the surfaces of mast cells and basophils, leading to Stage Two, acute inflammation.
Stage 2: The Chemical Cascade Begins
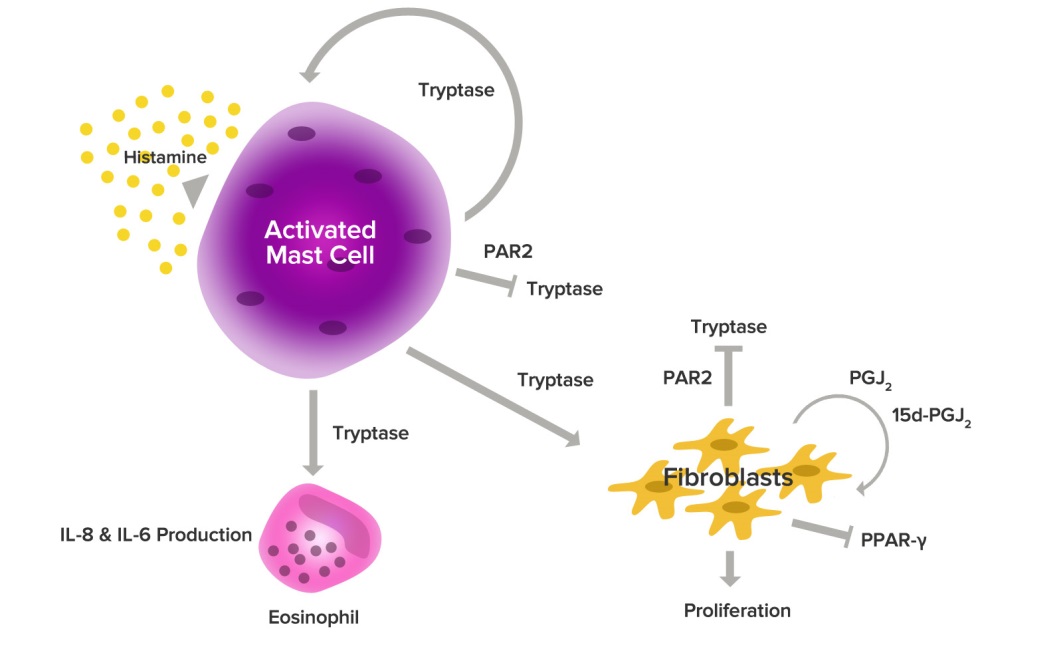
Stage 2 begins when the prepared immune system encounters the allergen at a future date. This time the immune system goes into action as the IgE antibodies bind to the allergen. This results in the activation of a chemical cascade as mast cells begin to ��degranulate�� and release histamine and other inflammatory chemicals �C cytokines, interleukins, leukotrienes, andprostaglandins �C into the surrounding tissues.
Histamine
Histamine dilates blood vessels, causing localized redness and swelling due to the release of fluids. Histamine also constricts bronchial tubes, impairs breathing, stimulates production of mucus in the respiratory system, and irritates nerve endings, causing itching and pain. Histamine is responsible for many common allergic symptoms, such as sneezing, itching, sinus congestion, wheezing, coughing, shortness of breath, and skin swelling, hives or rashes.
Leukotrienes
In addition to directly contributing to inflammation, histamine also initiates the release of leukotrienes (leukotrienes B4, C4, D4 and E4). These leukotrienes, especially leukotriene D4, are ten times more potent than histamine. In addition to constricting bronchial muscles, leukotrienes also act on blood vessels, causing them to become leaky and resulting in skin eruptions and swelling.
Prostaglandins
Unlike histamine, which is produced in both mast cells and basophils, prostaglandins are only released by the mast cells. One prostaglandin in particular, D2 (PGD2), is an even more potent bronchoconstrictor than histamine, though less so than the leukotrienes. Elevated PGD2 levels have been measured in secretions aspirated from the lungs of asthmatics and in nasal secretions from patients with nasal allergies.
Stage 3: Prolonged Immune Activation
Stage 3, or the ��late phase�� response is characterized by prolonged immune activation, usually occurring 2 to 24 hours after the initial allergic response. This late phase is characterized by the influx of additional inflammatory cells, as mast cells and neighboring tissues synthesize additional molecules that induce circulating basophils, eosinophils, and other cells to migrate into affected tissues. This generates a new wave of symptoms as the newly recruited cells begin secreting chemicals of their own to sustain the inflammatory process, resulting in local tissue damage. These late-phase inflammatory chemicals include additional immune mediators �C leukotrienes, prostaglandins, thromboxanes, and platelet activating factors �C that further aggravate the progression towards a state of chronic inflammation.
Cytokines
Several cytokines have been shown to play an important role in the regulation of IgE synthesis and the accumulation of eosinophils �C white blood cells responsible for combating infection and parasites �C during allergic reactions. One cytokine, interleukin 4 (IL-4), has been proven to be essential for promoting the production of IgE antibodies. Another cytokine, interleukin 5 (IL-5), also plays a key role in the maturation, activation and survival of eosinophils (increased numbers of eosinophils in blood and tissues is a characteristic feature of allergic disease).Tumor necrosis factor alpha (TNF alpha) is another cytokine that is produced rapidly during an allergic reaction. TNF-alpha regulates the secretion of additional cytokines to further attract and activate eosinophils while promoting the accumulation of inflammatory cells at the onset of the allergic reaction.Natural Allergy Relief | Nutrition Review
https://nutritionreview.org/2013/04/natural-allergy-relief/��
Janournal of Immunology, July 1, 2011
Mast Cells and Neutrophils Release IL-17 through Extracellular Trap Formation in Psoriasis
*Department of Dermatology, University of Michigan, Ann Arbor, MI 48109; and
†Division of Rheumatology, Department of Internal Medicine, University of Michigan, Ann Arbor, MI 48109
IL-17 and IL-23 are known to be absolutely central to psoriasis pathogenesis because drugs targeting either cytokine are highly effective treatments for this disease. The efficacy of these drugs has been attributed to blocking the function of IL-17�Cproducing T cells and their IL-23�Cinduced expansion. However, we demonstrate that mast cells and neutrophils, not T cells, are the predominant cell types that contain IL-17 in human skin. IL-17+ mast cells and neutrophils are found at higher densities than IL-17+ T cells in psoriasis lesions and frequently release IL-17 in the process of forming specialized structures called extracellular traps. Furthermore, we find that IL-23 and IL-1�� can induce mast cell extracellular trap formation and degranulation of human mast cells. Release of IL-17 from innate immune cells may be central to the pathogenesis of psoriasis, representing a fundamental mechanism by which the IL-23�CIL-17 axis mediates host defense and autoimmunity.
Much attention in recent years has focused on the production of IL-17A and IL-17F, often referred to together as IL-17, by a subset of T cells called Th17 cells (1). The differentiation of Th17 cells is supported by IL-6 and TGF-��, whereas IL-23, IL-1��, and IL-21 promote their expansion (2�C4). Interestingly, polymorphisms at loci encoding components of IL-23 and its receptor��IL23A, IL12B, and IL23R��have been associated with an increased risk of developing psoriasis (5�C9). Psoriasis lesions contain increased amounts of IL-17 mRNA and increased numbers of Th17 cells (2, 10, 11). Similarly, increased tissue expression of IL-17 and numbers of Th17 cells are seen in rheumatoid arthritis, Crohn��s disease, autoimmune uveitis, lupus erythematosus, ankylosing spondylitis, asthma, and multiple sclerosis (12). The pathophysiologic relevance of the IL-23�CIL-17 axis in autoinflammatory disease is highlighted by the clinical effectiveness of Abs targeting IL-23/IL-12 p40 and IL-17 in treating psoriasis as well as the other aforementioned diseases (13�C16).
Although IL-17 production by T cells is widely studied, it is increasingly appreciated that diverse types of innate immune cells also can produce IL-17 (17). Recent studies demonstrate that IL-17+ mast cells and neutrophils can be found in complicated atherosclerotic plaques (18). Similarly, IL-17+ mast cells are also present in inflamed synovium of rheumatoid arthritis (19). Mast cells, particularly a subset containing tryptase and chymase (MCTCs), are enriched in the papillary dermis of psoriasis lesions (20, 21). Mast cells frequently degranulate in early eruptive and recurring psoriasis lesions and have been described as ��ghost cells�� (22�C24). Interestingly, mast cell numbers are decreased in psoriasis lesions after successful treatment with anthralin, psoralen plus UVA light therapy, or cyclosporine (25�C27). Neutrophils also are enriched in psoriasis lesions, especially in the epidermis where they aggregate in Munro��s microabscesses (MMs) in the stratum corneum and spongiform pustules of Kogoj (SPKs) in the stratum spinosum (28). Although the precise function of neutrophils is unknown in psoriasis, a critical pathogenic role is supported by case reports of psoriasis remission during drug-induced agranulocytosis and its reappearance after the normalization of neutrophil numbers (29). Furthermore, razoxane, a drug effective against all forms of psoriasis and psoriatic arthritis, causes a dose-dependent depression of neutrophil counts (30). Very recently, a study indicated that mast cells and neutrophils contain IL-17 in psoriasis plaques of three patients (31). However, the significance of these numbers relative to normal-appearing skin and the mechanism of IL-17 release by innate immune cells in psoriasis remain unknown.
IL-17 orchestrates innate immune responses against extracellular pathogens by inducing expression of antimicrobial peptides (AMPs) and neutrophil-tropic chemokines CXCL1, CXCL2, and IL-8 (32�C35). The same AMPs and chemokines are found at extremely high levels in psoriatic epidermis (36�C38). Not surprisingly, mice and humans with deficits in IL-17 production or signaling are highly susceptible to infection with extracellular bacteria and fungi (39�C42). Similarly, effective antimicrobial activity by neutrophils and mast cells depends on the formation of structures called extracellular traps (ETs), termed NETs and MCETs, respectively (43�C45). ETs are formed through a specialized process of cell death termed ETosis (46), where chromatin extends into fine, weblike threads to which proteins are bound. In particular, NETs can contain myeloperoxidase (MPO), proteinase 3, and AMPs such as cathelicidin (LL-37) (47). The process of NET formation (NETosis) can be triggered by extracellular bacteria and fungi or their components (48, 49). MCETs contain tryptase, LL-37, and chromatin (45), forming in response to bacteria, H2O2, or PMA (45, 48). In humans, NETs have been visualized in physiologic host responses to infections (44, 50) and have been implicated in the pathology of anti-neutrophil cytoplasmic Ab-induced vasculitis (51). Additionally, a recent study showed that lupus nephritis is associated with an inability to degrade NETs in blood (52). Furthermore, although MCETs have been studied in a human mast cell line and mouse bone marrow-derived mast cells (45), it is unclear whether the process of MCET formation (MCETosis) occurs in human tissue under normal or pathologic conditions.
To understand the complex pathophysiology of psoriasis, it is imperative to define the precise cellular sources of IL-17 and the mechanisms mediating IL-17 release. Therefore, we investigate the production of IL-17 by innate immune cells in psoriasis and explore a potential role for ETs formed by these cells in human skin. Surprisingly, we observe that most IL-17+ cells in normal and psoriatic skin are mast cells, not T cells. Additionally, neutrophils in well-developed psoriasis lesions also express IL-17. We observe that mast cells and neutrophils release IL-17 into the skin though ETosis as well as conventional degranulation. Furthermore, a subtype of human neutrophils, low-density granulocytes (LDGs), isolated from psoriatic blood are increased in psoriatic compared with control blood. Interestingly, mast cells readily form ETs with resultant IL-17 release in control human skin explants treated with IL-23 and IL-1��, suggesting a novel mechanism driving ETosis and IL-17 release from mast cells. These findings suggest that mast cells and neutrophils may play more prominent roles than previously appreciated in psoriasis and similar diseases by releasing IL-17 through ET formation.
http://www.jimmunol.org/content/187/1/490��
Mast Cell Responses to Cell Surface TLRs
Acting via TLR4, LPS caused IL-6, IL-13, and TNF�� secretion from murine BMMC (McCurdy et al., 2001; Supajatura et al., 2001) and a later study found secretion of IL-5 and IL-10 upon LPS stimulation via TLR4 (Masuda et al., 2002). In addition to these cytokines, LPS stimulation of murine BMMC and fetal skin-derived mast cells (FSDMC) also caused the secretion of the chemokines CCL3/MIP-1�� and CXCL2/MIP-2 (Matsushima et al., 2004; Figure 1).
FIGURE 1
www.frontiersin.org
FIGURE 1. Mast cell secretory responses to TLR ligation. A diagram showing the molecules secreted by mast cells upon TLR ligation. The cytokines, chemokines, and lipid mediators released upon TLR ligand stimulation are summarized for murine BMMC, PCDMC, FSDMC, and human mast cells. Where there is discrepancy in the literature, molecules are shown in grey. *indicates instances where it has been demonstrated with the use of TLR-deficient cells or blocking antibodies that the ligand is acting via the indicated receptor.
Differences between the cytokines produced upon TLR4 and TLR2 stimulation have been observed: LPS caused murine BMMC to secrete TNF��, IL-6, IL-13, and IL-1�� via TLR4; while peptidoglycan (PGN) causes the secretion of TNF�� and IL-6, in addition to the Th2 cytokines, IL-4, IL-5, and IL-13 via TLR2 (Supajatura et al., 2002; Figure 1). In rat peritoneal mast cells, both PGN and LPS resulted in cysteinyl leukotriene production, but the response to PGN was greater (Wierzbicki and Brzezinska-Blaszczyk, 2009). Taken together, these findings suggest that mast cells release a wider variety of mediators in response to PGN than LPS. This appears not to be the case in macrophages, where stimulation with LPS or PGN has been shown to lead to an up-regulation of similar mRNAs (Wang et al., 2000).
Murine peritoneal cell-derived mast cells (PCDMC) responded more potently to TLR agonists than BMMC and it is suggested that the PCDMC are more mature than BMMC, and this increased maturity underlines their increased ability to respond to TLR stimulation (Mrabet-Dahbi et al., 2009). LTA and MALP-2 treatment of PCDMC resulted in IL-1, IL-6, IL-17, GM-CSF, IL-10, TNF��, and IFN�� production, while LPS caused only IL-6, GM-CSF, IL-10, and TNF�� secretion from PCDMC (Mrabet-Dahbi et al., 2009). Of these three agonists, only LTA-induced PGD2 production in PCDMC (Mrabet-Dahbi et al., 2009; Figure 1).
In human cord blood-derived mast cells (CBMC), stimulation with zymosan or PGN caused GM-CSF, IL-1��, LTB4, and LTC4 production (Olynych et al., 2006). Another study also identified differences between the mediators released upon different TLR ligand stimulation: PGN, zymosan, and Pam3Cys caused GM-CSF and IL-1�� secretion whereas LPS did not; and PGN and zymosan treatment led to the production of LTC4 unlike Pam3Cys treatment (McCurdy et al., 2003). Human mast cells cultured from CD34+ progenitors isolated from blood (PBDMC) stimulated with LPS produced significant amounts of TNF��, whereas PGN induced IL-1��, GM-CSF, IL-5, and cysteinyl leukotriene in addition to TNF�� (Kulka et al., 2004; Figure 1). Therefore, as has been shown in murine mast cells, stimulation via TLR2 results in a greater range of mediator production than stimulation via TLR4.
Pre-treatment of mast cells with cytokines has been shown to enhance the response of the cells to TLR ligands (Okumura et al., 2003; Varadaradjalou et al., 2003). In one study, LPS only induced TNF�� production after the CBMC had been incubated with IL-4, whereas even untreated cells were able to respond to PGN (Varadaradjalou et al., 2003). It is not clear why the cells in this study were unable to respond to the TLR4 agonist without TNF�� pre-treatment (Varadaradjalou et al., 2003), unlike human PBDMC (Kulka et al., 2004). Human lung mast cells and PBDMC responded to LPS by secreting TNF�� but this response, and TLR4 expression, was increased by pre-treatment with IFN�� (Okumura et al., 2003). This group also noticed CCL1 and IL-5 production in LPS-treated lung mast cells but not PBDMC, and gene array analysis showed that LPS caused the up-regulation of a variety of genes including a protease, several cytokines, chemokines, receptors, and STAT5a (Okumura et al., 2003).
Peptidoglycan has been demonstrated to induce migration of peritoneal rat mast cells after a short treatment with TNF�� (Brzezinska-Blaszczyk and Rdzany, 2007), and in a later publication, LPS and PGN both caused migration of the cells after treatment with IL-6 or CCL5/RANTES, respectively (Wierzbicki and Brzezinska-Blaszczyk, 2009). The mechanism behind these effects is as yet unknown, but it has been suggested that IL-6 and CCL5/RANTES may modulate TLR expression on the mast cells (Wierzbicki and Brzezinska-Blaszczyk, 2009). The ability of TLR agonists to cause mast cell migration in vivo would allow PAMPs or endogenous TLR ligands produced upon tissue damage to recruit mast cells to sites of infection or inflammation.
TLR5 expression has been more readily detected on human than murine mast cells (see Table 1) and human PBDMC respond to flagellin (a TLR5 ligand) by secreting IL-1�� and TNF��, demonstrating that the receptor is functional on these cells (Kulka et al., 2004; Figure 1). To our knowledge, flagellin has not been shown to cause cytokine secretion from murine mast cells, in agreement with the lack of detectable expression of TLR5 on the cells (McCurdy et al., 2001; Supajatura et al., 2001; Ikeda and Funaba, 2003; Matsushima et al., 2004).Frontiers | TLR signaling in mast cells: common and unique features | Immunology
https://www.frontiersin.org/articles/10.3389/fimmu.2012.00185/full#:~:text=Mast%20cells%20respond%20to%20TLR%20ligands%20by%20secreting,also%20cause%20degranulation%2C%20although%20this%20finding%20is%20contentious.��
Apr 21, 2015
Mast Cells Are a Source of IL-17 and IL-22 in Psoriasis and Atopic Dermatitis
Craig A. Elmets, MD reviewing Mashiko S et al. J Allergy Clin Immunol 2015 Mar 17
Mast Cells Are a Source of IL-17 and IL-22 in Psoriasis and Atopic Dermatitis
https://www.jwatch.org/na37519/2015/04/21/mast-cells-are-source-il-17-and-il-22-psoriasis-and-atopic��
J Allergy Clin Immunology. 2015 Aug;
Human mast cells are major IL-22 producers in patients with psoriasis and atopic dermatitis
Immunoregulation Laboratory, Centre de Recherche du Centre Hospitalier de l'Universit�� de Montr��al (CRCHUM), Montreal, Quebec, Canada.
2Center for Innovative and Translational Medicine, Kochi University Medical School, Nankoku, Kochi, Japan.
3Innovaderm Research, Montreal, Quebec, Canada.
4Immunoregulation Laboratory, Centre de Recherche du Centre Hospitalier de l'Universit�� de Montr��al (CRCHUM), Montreal, Quebec, Canada. Electronic address: m.sarfati@umontreal.ca.
Background: Psoriasis is a systemic inflammatory disease in which IL-17 and IL-22 levels are markedly increased in the skin and blood. The prevalent concept, using skin cells that are isolated from psoriatic plaques and examined after cell expansion and in vitro stimulation, is that IL-17 and IL-22 production essentially results from T cells and the rare type 3 innate lymphoid cells.
Objective: We sought to examine the cellular source of IL-17A and IL-22 at the protein and transcriptional single-cell level immediately after ex vivo skin cell isolation from psoriatic plaques.
Methods: Skin biopsy specimens were collected from patients with psoriasis, as well as from patients with atopic dermatitis. Cell suspensions were prepared by combining mild enzymatic digestion and mechanical dissociation and analyzed for cytokine expression without prior in vitro culture and stimulation. Expression of IL-17 and IL-22 was quantified at the protein and mRNA single-cell level by using flow cytometry.
Results: IL-22 is predominantly expressed by CD3(-)c-Kit(+) cells relative to CD3(+) T cells in lesional skin of patients with psoriasis and patients with atopic dermatitis. Strikingly, we identified c-Kit(+)Fc��RI(+) mast cells as major IL-22 producers. The proportion of mast cells that produce IL-22 ranges from 20% to 80% in patients with psoriasis or those with atopic dermatitis. Skin mast cells express IL-22 and IL-17 mRNA. Conversely, IL-17-producing T cells outnumber IL-17-producing mast cells, which also express IL-17 receptor.
Conclusion: Human skin mast cells are previously unrecognized IL-22 producers. We further established that skin mast cells express IL-17. Thus mast cells might play an important role in the physiopathology of chronic inflammatory skin disorders.
Keywords: IL-17; IL-22; Mast cells; T cells; atopic dermatitis; psoriasis.Human mast cells are major IL-22 producers in patients with psoriasis and atopic dermatitis - PubMed
https://pubmed.ncbi.nlm.nih.gov/25792465/��
J Invest Dermatology. 2013 Apr;
CD8(+) T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-��, IL-13, IL-17, and IL-22
1Department of Dermatology, University Medical Center Utrecht, Utrecht, The Netherlands. dirkjanhijnen@gmail.com
Although CD4(+) T cells are known to contribute to the pathology of atopic dermatitis (AD) and psoriasis, the role of CD8(+) T cells in these diseases remains poorly characterized. The aim of this study was to characterize the cytokine production of T cells from AD and psoriasis skin. We found that CD4(+) T cells isolated from AD skin were largely Th2 (T helper type 2) biased, in agreement with prior reports. However, we also observed large numbers of CD8(+) T cells producing IL-13, IFN-��, and IL-22. We observed increased numbers of CD8(+) T cells isolated from AD skin, and immunohistochemistry studies confirmed the presence of CD8(+) T cells in the dermis and epidermis of AD skin lesions. Surprisingly, T-cell cytokine production was similar in the lesional and nonlesional skin of patients with AD. T cells from psoriatic lesional skin predominantly produced IFN-��, IL-17, and IL-22, in agreement with prior studies. However, in addition to Th17 cells, we observed high percentages of CD8(+) T cells that produced both IL-22 and IL-17 in psoriatic skin lesions. Our findings demonstrate that CD8(+) T cells are a significant and previously unappreciated source of inflammatory cytokine production in both AD and psoriasis.CD8(+) T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-��, IL-13, IL-17, and IL-22 - PubMed
https://pubmed.ncbi.nlm.nih.gov/23223131/��
Intratumor IL-17-positive mast cells are the major source of the IL-17 that is predictive of survival in gastric cancer patients
Department of Gastrointestinal Surgery, the First Affiliated Hospital, Medical College, Zhejiang University, Hangzhou, China.
Interleukin-17 (IL-17) is prevalent in tumor tissue and suppresses effective anti-tumor immune responses. However, the source of the increased tumor-infiltrating IL-17 and its contribution to tumor progression in human gastric cancer remain poorly understood. In this study, we enrolled 112 gastric cancer patients, immunofluorescence was used to evaluate the colocalization of CD3, CD4, CD56, CD20, CD68, and mast cell tryptase (MCT) with IL-17. Immunohistochemistry was used to evaluate the distribution of microvessel density (CD34), CD66b(+), CD68(+), and FoxP3(+) cells in different microanatomical areas. Prognostic value was determined by Kaplan-Meier analysis and a Cox regression model. The results showed that mast cells, but not T cells or macrophages, were the predominant cell type producing IL-17 in gastric cancer. Significant positive correlations were detected between densities of mast cell-derived IL-17 and microvessels, neutrophils, and regulatory T cells (Tregs). Furthermore, we found that the majority of vascular endothelial cells expressing Interleukin-17 receptor (IL-17R). Kaplan-Meier analysis revealed that increasing intratumor infiltrated mast cells and IL-17(+) cells, as well as MCT(+) IL-17(+) cells, were significantly associated with worse overall survival. These findings indicated that mast cells were the major source of IL-17 in gastric cancer, and intratumor IL-17 infiltration may have promoted tumor progression by enhancing angiogenesis in the tumor microenvironment through the axis of IL-17/IL-17R. IL-17-positive mast cells showed a prognostic factor in gastric cancer, indicating that immunotherapy targeting mast cells might be an effective strategy to control intratumor IL-17 infiltration, and consequently reverse immunosuppression in the tumor microenvironment, facilitating cancer immunotherapy.Intratumor IL-17-positive mast cells are the major source of the IL-17 that is predictive of survival in gastric cancer patients - PubMed
https://pubmed.ncbi.nlm.nih.gov/25197971/��
Medicine (Baltimore).2016 Mar;
Mast Cells Comprise the Major of Interleukin 17-Producing Cells and Predict a Poor Prognosis in Hepatocellular Carcinoma
1From the Department of Radiology (J-FT, X-HY, J-SJ), Lishui Hospital Affiliated to Zhejiang University; Department of Infection Diseases (H-YP); Department of Tumor Surgery (JL), the First Affiliated Hospital Zhejiang University; and Department of Cardiology (HZ), Zhejiang Provincial People's Hospital, Hangzhou, Zhejiang Province, China.
IL-17 and IL-17-producing cells have been found in many types of human cancers and murine models. However, the source of tumor-infiltrating IL-17 and IL-17-producing cells in HCC and the prognostic values remain poorly understood. A total of 57 HCC patients were enrolled in this study, and immunofluorescence double stain was used to evaluate the colocalization of CD3 T cells, CD4 T cells, CD56 NK cells, CD20 B cells, CD68 Macrophages, and MCT mast cells with IL-17. The prognostic value of IL-17-producing cells was evaluated by Kaplan-Meier analysis and Cox regression model. MCT mast cells, but not other cells, were the predominant IL-17-producing cell type. Overall survival analysis revealed that the increasing intratumoral-infiltrated MCT mast cells were significantly associated with poor prognosis. Immunofluorescence double stain showed a positive correlation between the number of MCT mast cells and MCVs. These findings indicated the major IL-17-producing cells in HCC were MCT mast cells and these cells infiltration may promote tumor progression by angiogenesis. Increased MCT mast cells was associated with a poor prognosis, indicating therapy targeting MCT mast cells might be an effective strategy in controlling intratumor IL-17 infiltration and MCVs.Mast Cells Comprise the Major of Interleukin 17-Producing Cells and Predict a Poor Prognosis in Hepatocellular Carcinoma - PubMed
https://pubmed.ncbi.nlm.nih.gov/27043690/��
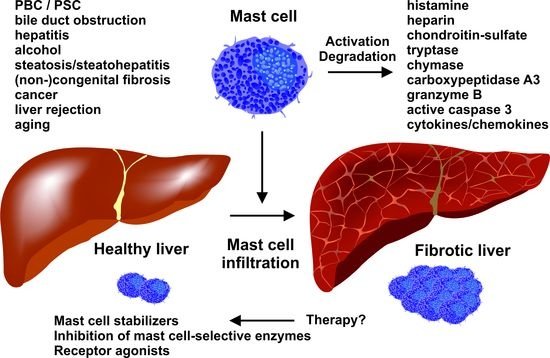
Mast Cells in Liver Fibrogenesis
1 Institute of Molecular Pathobiochemistry, Experimental Gene Therapy and Clinical Chemistry (IFMPEGKC), University Hospital, RWTH Aachen University, D-52074 Aachen, Germany
2 Department of Internal Medicine III, University Hospital, RWTH Aachen University, D-52074 Aachen, Germany
3 Institute of Biochemistry and Molecular Immunology, Medical Faculty, RWTH Aachen University, D-52074 Aachen, Germany
Mast cells (MCs) are immune cells of the myeloid lineage that are present in the connective tissue throughout the body and in mucosa tissue. They originate from hematopoietic stem cells in the bone marrow and circulate as MC progenitors in the blood. After migration to various tissues, they differentiate into their mature form, which is characterized by a phenotype containing large granules enriched in a variety of bioactive compounds, including histamine and heparin. These cells can be activated in a receptor-dependent and -independent manner. Particularly, the activation of the high-affinity immunoglobulin E (IgE) receptor, also known as Fc��RI, that is expressed on the surface of MCs provoke specific signaling cascades that leads to intracellular calcium influx, activation of different transcription factors, degranulation, and cytokine production. Therefore, MCs modulate many aspects in physiological and pathological conditions, including wound healing, defense against pathogens, immune tolerance, allergy, anaphylaxis, autoimmune defects, inflammation, and infectious and other disorders. In the liver, MCs are mainly associated with connective tissue located in the surrounding of the hepatic arteries, veins, and bile ducts. Recent work has demonstrated a significant increase in MC number during hepatic injury, suggesting an important role of these cells in liver disease and progression. In the present review, we summarize aspects of MC function and mediators in experimental liver injury, their interaction with other hepatic cell types, and their contribution to the pathogenesis of fibrosis.Cells | Free Full-Text | Mast Cells in Liver Fibrogenesis
https://www.mdpi.com/2073-4409/8/11/1429��
IgE-mediated mast cell activation promotes inflammation and cartilage destruction in osteoarthritis
VA Palo Alto Health Care System, United States; Stanford University School of Medicine, United States; University of Padova, Italy; Virginia Commonwealth University School of Medicine, United States
Osteoarthritis is characterized by articular cartilage breakdown, and emerging evidence suggests that dysregulated innate immunity is likely involved. Here, we performed proteomic, transcriptomic, and electron microscopic analyses to demonstrate that mast cells are aberrantly activated in human and murine osteoarthritic joint tissues. Using genetic models of mast cell deficiency, we demonstrate that lack of mast cells attenuates osteoarthritis in mice. Using genetic and pharmacologic approaches, we show that the IgE/Fc��RI/Syk signaling axis is critical for the development of osteoarthritis. We find that mast cell-derived tryptase induces inflammation, chondrocyte apoptosis, and cartilage breakdown. Our findings demonstrate a central role for IgE-dependent mast cell activation in the pathogenesis of osteoarthritis, suggesting that targeting mast cells could provide therapeutic benefit in human osteoarthritis.IgE-mediated mast cell activation promotes inflammation and cartilage destruction in osteoarthritis | eLife
https://elifesciences.org/articles/39905��