肠道是 健康之本,百病之源 The Gut is the base of Health and The Root of Disease
1. zinc
2. Omega-3,ALA, EPA, DHA
3. diarrhea:calcium + vitamin D
a Prominent health conditions with both biologic age and chronic inflammation as central risk factors. b Prominent health conditions with evidence linking them to gut dysbiosis. Note the similarities between the conditions associated with aging and inflammation and those associated with gut dysbiosis
Simplified schematic outlining the potential relationships among health risk factors, gut dysbiosis, inflammation, and age-related disease—as well as potential interventions for attenuating gut-associated inflammation
(Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease | Microbiome | Full Text
https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-017-0296-0
Control Secretory IgA & Keep A Happy Gut — MyBioHack | Unlock Your Maximum Potential
https://mybiohack.com/blog/siga-secretory-iga-glutamine-tgf-il-6-10-microbiome-homeostasis-inflammation-high-low
Control Secretory IgA & Keep A Happy Gut — MyBioHack | Unlock Your Maximum Potential
https://mybiohack.com/blog/siga-secretory-iga-glutamine-tgf-il-6-10-microbiome-homeostasis-inflammation-high-lowM Cells (Microfold Cells)
Microfold or M cells are epithelial cells embedded in the epithelium of mucosal tissues such as intestine, lung, and nasopharynx. Their name derives from the characteristic morphology of their apical or lumen-facing surface which has few if any microvilli.
The importance of M cells is that they are a critical player in the mucosal immune response and immune surveillance. The have have a unique ability to take up a variety of antigens - bacteria, viruses, parasites and proteins - from the lumen, transport them by transcytosis across the cell and deliver them to immune cells lying underneath, particularly dendritic cells. In other words, M cells are antigen-delivery cells for the immune system in places like the intestine and lung, the first step in eliciting a mucosal immune response. The capture of lumenal antigens occurs through electrostatic interactions and by binding to a number of adhesion molecules on the surface of the M cell.
M cells are most abundant in the epithelium overlying lymphoid follicles such as Peyer's patches in the intestine. There are additional types of M cells such as villous M cells interspersed among entercytes over villi in the small intestine.
The ability of M cells to deliver antigens to the mucosal immune system has led to the concept of exploiting the cells for delivery of vaccines targeting mucosal immune responses, which is an active area of research.
Paradoxically, although M cells are important in generating an effective mucosal immune response to protect against many pathogens, they also serve as an entry portal for a number of pathogens that can be present in the intestinal lumen. Examples include bacteria such as Brucella, Yersinia, and Salmonella species, viruses like reoviruses and poliovirus, and prions. In these cases, the pathogen is taken up and sometimes replicates in M cells, and is then transferred across the cell to induce a systemic infection.M Cells
http://www.vivo.colostate.edu/hbooks/pathphys/digestion/smallgut/mcells.html
Relationship between microbiota diversity, epithelial Integrity, and inflammation in disease state
The lysis of gram-negative bacteria causes them to release lipopolysaccharide (LPS; endotoxin) from the outer membrane of their cell wall. The LPS binds to a LPS-binding protein circulating in the blood and this complex, in turn, binds to a receptor molecule (CD14) found on the surface of body defense cells called macrophages. This is thought to promote the ability of the toll-like receptor TLR-4 to respond to the LPS, triggering the macrophage to release various defense regulatory chemicals called cytokines, including IL-1, IL-6, IL-8, TNF-alpha, and PAF. The cytokines then bind to cytokine receptors on target cells and initiate inflammation and activate both the complement pathways and the coagulation pathway. (LPS, lipopolysaccharide; IL-1, interleukin-1; IL-6, interleukin-6; IL-8, interleukin-8, TNF-alpha, tumor necrosis factor-alpha; PAF, platelet-activating factor.) This will be discussed in greater detail under Bacterial Pathogenicity.
Another cell surface PRR is CD14. CD14 is found on monocytes, macrophages, and neutrophils and promotes the ability of TLR-4 to respond to LPS. LPS typically binds to LPS-binding protein in the plasma and tissue fluid. The LPS-binding protein promotes the binding of LPS to the CD14 receptors. At that point the LPS-binding protein comes off and the LPS-CD14 bind to TLR-4. Interaction of LPS and CD14 with TLR-4 leads to an elevated synthesis and secretion of inflammatory cytokines such as IL-1, IL-6, IL-8, TNF-alpha, and platelet-activating factor (PAF). These cytokines then bind to cytokine receptors on target cells and initiate inflammation and activate both the complement pathways and the coagulation pathway (see Fig. 7).Innate Immunity
Early Induced Innate Immunity: Pattern-Recognition Receptors (PRRs) and Danger-Recognition Receptors (DRRs)
Fundamental Statement for this Softchalk Lesson:
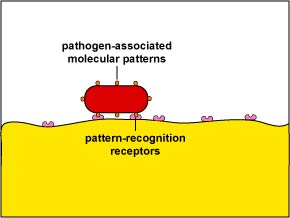
1. Early induced innate immunity begins 4 - 96 hours after exposure to an infectious agent and involves the recruitment of defense cells as a result of pathogen-associated molecular patterns or PAMPS binding to pattern-recognition receptors or PRRs and danger-associated molecular patterns or DAMPs binding to danger-recognition receptors or DRRs.
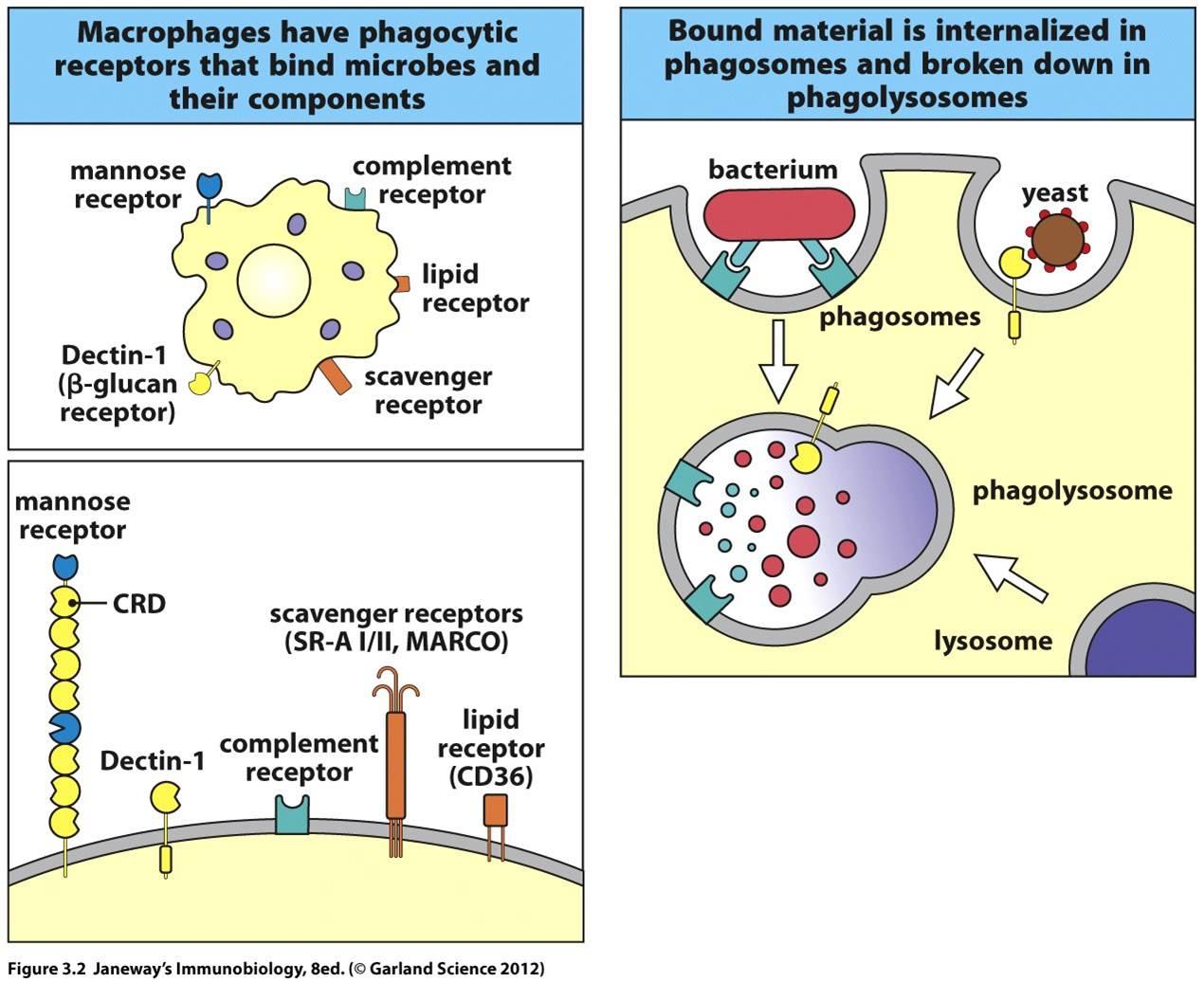
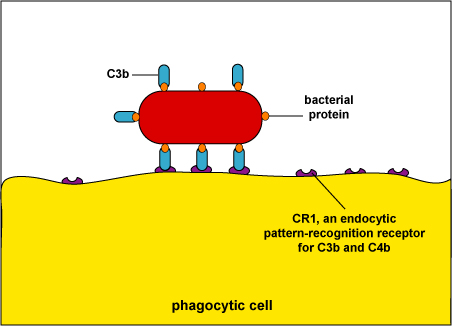
2. Endocytic pattern-recognition receptors are found on the surface of phagocytes and promote the attachment of microorganisms to phagocytes leading to their subsequent engulfment and destruction. They include mannose receptors, scavenger receptors, and opsonin receptors.
3. Binding of microbial PAMPs to signaling PRRs promotes the production of inflammatory cytokines, antiviral cytokines called type-1 interferons (IFN), chemotactic factors, and antimicrobial peptides. They include toll-like receptors (TLRs) and NODs.
4. PRRs found on the surface of the body's cells typically bind to surface PAMPs on microbes and stimulate the production of inflammatory cytokines.
5. PRRs found within cellular phagolysosomes (endosomes) typically detect nucleic acid PAMPs released during the phagocytic destruction of viruses and stimulate the production of antiviral cytokines called type-1 interferons.
6. PRRs and DRRs found within the cytoplasm of host cells typically trigger the formation of multi-protein complexes called inflammasomes which, in turn, triggers the formation of inflammatory cytokines and can also leads to an inflammatory response-induced cell suicide called pyroptosis.
7. PRRs circulating in the blood and tissue fluid activate the complement pathways and may function as opsonins.
早期诱导的先天免疫:模式识别受体(PRR)和危险识别受体(DRR):1.早期诱导的先天免疫开始于暴露于传染剂后4-96小时,由于病原体相关分子模式或PAMPS与模式识别受体或PRR结合以及危险相关分子模式或DAMP与危险识别受体或DRR结合。
2.在吞噬细胞的表面发现内吞模式识别受体,并促进微生物与吞噬细胞的附着,导致其随后被吞噬和破坏。它们包括甘露糖受体,清除剂受体和调理素(opsonins)受体。
3.微生物PAMP与信号PRR的结合会促进炎症细胞因子,称为1型干扰素(IFN)的抗病毒细胞因子,趋化因子和抗菌肽的产生。它们包括收费型受体(TLR)和NOD。
4.在人体细胞表面发现的PRR通常与微生物表面的PAMP结合并刺激炎性细胞因子的产生。
5.在细胞吞噬溶酶体(内体)中发现的PRR通常检测病毒吞噬破坏过程中释放的核酸PAMP,并刺激称为1型干扰素的抗病毒细胞因子的产生。
6.在宿主细胞质中发现的PRR和DRR通常会触发称为炎症小体的多蛋白复合物的形成,进而触发炎症性细胞因子的形成,还可能导致炎症反应诱导的细胞自杀,称为焦磷酸化。
7.在血液和组织液中循环的PRR激活补体途径,并可能起调理素的作用。
Early Induced Innate Immunity: Pattern-Recognition Receptors (PRRs) and Danger-Recognition Receptors (DRRs)
Early induced innate immunity begins 4 - 96 hours after exposure to an infectious agent and involves the recruitment of defense cells as a result of pathogen-associated molecular patterns or PAMPS binding to pattern-recognition receptors or PRRs. These recruited defense cells include:
phagocytic cells: leukocytes such as neutrophils, eosinophils, and monocytes; tissue phagocytic cells in the tissue such as macrophages;
cells that release inflammatory mediators: inflammatory cells in the tissue such as macrophages and mast cells; leukocytes such as basophils and eosinophils; and
natural killer cells (NK cells).
Unlike adaptive immunity, innate immunity does not recognize every possible antigen. Instead, it is designed to recognize molecules shared by groups of related microbes that are essential for the survival of those organisms and are not found associated with mammalian cells. These unique microbial molecules are called pathogen-associated molecular patterns or PAMPS and include LPS from the gram-negative cell wall, peptidoglycan and lipotechoic acids from the gram-positive cell wall, the sugar mannose (a terminal sugar common in microbial glycolipids and glycoproteins but rare in those of humans), bacterial and viral unmethylated CpG DNA, bacterial flagellin, the amino acid N-formylmethionine found in bacterial proteins, double-stranded and single-stranded RNA from viruses, and glucans from fungal cell walls. In addition, unique molecules displayed on stressed, injured, infected, or transformed human cells also be recognized as a part of innate immunity. These are often referred to as danger-associated molecular patterns or DAMPs.
Most body defense cells have pattern-recognition receptors or PRRs for these common PAMPS (see Fig. 1) and so there is an immediate response against the invading microorganism. Pathogen-associated molecular patterns can also be recognized by a series of soluble pattern-recognition receptors in the blood that function as opsonins and initiate the complement pathways. In all, the innate immune system is thought to recognize approximately 103 of these microbial molecular patterns.早期诱导的先天免疫:模式识别受体(PRR)和危险识别受体(DRR)
早期诱导的先天免疫在接触感染剂后4-96小时开始,由于病原体相关的分子模式或PAMPS与模式识别受体或PRR结合,招募了防御细胞。这些招募的防御单位包括:
吞噬细胞:白细胞,例如嗜中性粒细胞,嗜酸性粒细胞和单核细胞;组织中的吞噬细胞组织,例如巨噬细胞;
释放炎性介质的细胞:组织中的炎性细胞,例如巨噬细胞和肥大细胞;白细胞,如嗜碱性粒细胞和嗜酸性粒细胞;和
自然杀伤细胞(NK细胞)。
与适应性免疫不同,先天免疫不能识别所有可能的抗原。取而代之的是,它被设计为识别由相关微生物群共享的分子,这些分子对于这些生物体的生存至关重要,并且未发现与哺乳动物细胞相关的分子。这些独特的微生物分子被称为病原体相关分子模式或PAMPS,包括来自革兰氏阴性细胞壁的LPS,来自革兰氏阳性细胞壁的肽聚糖和脂解酸,糖甘露糖(微生物糖脂和糖蛋白中常见的末端糖,但细菌和病毒未甲基化的CpG DNA,细菌鞭毛蛋白,细菌蛋白质中发现的氨基酸N-甲酰甲硫氨酸,病毒的双链和单链RNA以及真菌细胞壁的葡聚糖等。另外,在应激,受伤,感染或转化的人类细胞上展示的独特分子也被认为是先天免疫的一部分。这些通常称为与危险相关的分子模式或DAMP。
大多数人体防御细胞具有这些常见PAMPS的模式识别受体或PRR(见图1),因此可以对入侵的微生物立即做出反应。病原相关的分子模式也可以被血液中的一系列可溶性模式识别受体所识别,这些受体起调理素的作用并启动补体途径。总之,先天免疫系统被认为可以识别大约103种微生物分子模式。
Glycoprotein molecules known as pattern-recognition receptors are found on the surface of a variety of body defense cells. They are so named because they recognize and bind to pathogen-associated molecular patterns - molecular components associated with microorganisms but not found as a part of eukaryotic cells. These include bacterial molecules such as peptidoglycan, teichoic acids, lipopolysaccharide, mannans, flagellin, pilin, and bacterial DNA. There are also pattern-recognition molecules for viral double-stranded RNA (dsRNA) and fungal cell walls components such as lipoteichoic acids, glycolipids, mannans, and zymosan. Many of these pattern recognition receptors are known as toll-like receptors.
在各种人体防御细胞的表面发现了被称为模式识别受体的糖蛋白分子。之所以命名它们,是因为它们识别并结合与病原体相关的分子模式-与微生物相关的分子成分,但并未作为真核细胞的一部分被发现。这些包括细菌分子,例如肽聚糖,海藻酸,脂多糖,甘露聚糖,鞭毛蛋白,菌毛蛋白和细菌DNA。也存在病毒双链RNA(dsRNA)和真菌细胞壁成分(例如脂蛋白,糖脂,甘露聚糖和酵母聚糖)的模式识别分子。这些模式识别受体中有许多被称为收费样受体。
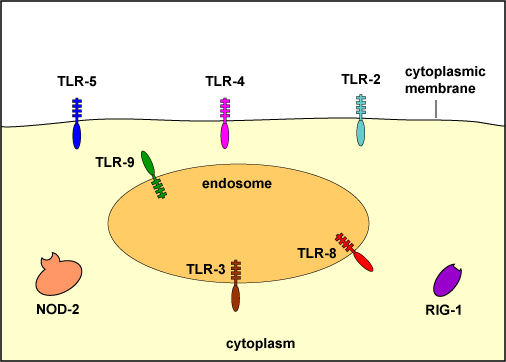
To recognize PAMPs such as those listed above, various body cells have a variety of corresponding receptors called pattern-recognition receptors or PRRs capable of binding specifically to conserved portions of these molecules. Cells that typically have pattern recognition receptors include macrophages, dendritic cells, endothelial cells, mucosal epithelial cells, and lymphocytes. Many pattern-recognition receptors are located on the surface of these cells where they can interact with PAMPs on the surface of microbes. Others PRRs are found within the phagolysosomes of phagocytes where they can interact with PAMPs located within microbes that have been phagocytosed. Some PRRs are found in the cytosol of the cell.
KEY
on the surface:
TLR-2 - recognizes peptidoglycan, bacterial lipoproteins, lipoteichoic acid, and porins
TLR-4 - recognizes lipopolysaccharide (LPS) from gram-negative cell wall, fungal mannans, viral envelope proteins, parasitic phospholipids, heat-shock proteins
TLR-5 - recognizes bacterial flagellin
within endosomes (phagolysosomes):
TLR-3 - recognizes viral double-stranded DNA
TLR-8 - recognizes viral single-stranded RNA
TLR-9 - recognizes viral and bacterial unmethylated CpG sequences
in the cytoplasm:
NOD-2 - recognizes muramyl dipeptide from bacterial peptidoglycan
RIG-1 - recognizes viral RNAMany pattern-recognition receptors are located on the surface of these cells where they can interact with PAMPs on the surface of microbes. Others PRRs are found within the phagolysosomes of phagocytes where they can interact with PAMPs located within microbes that have been phagocytosed. Some PRRs are found in the cytosol of the cell.
There are two functionally different major classes of pattern-recognition receptors: endocytic pattern-recognition receptors and signaling pattern-recognition receptors.
a. Endocytic (Phagocytic) Pattern-Recognition Receptors
Endocytic pattern-recognition receptors, also called phagocytic pattern-recognition receptors, are found on the surface of phagocytes and promote the attachment of microorganisms to phagocytes leading to their subsequent engulfment and destruction. They include:
1. Mannose receptors
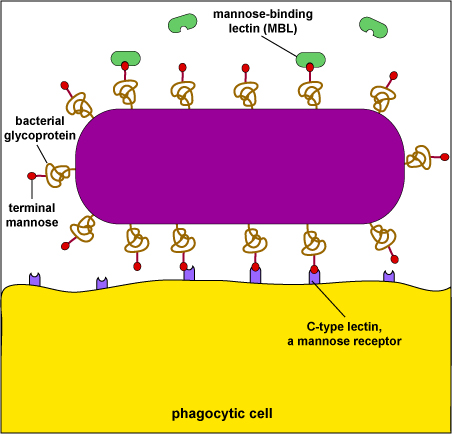
Mannose receptors on the surface of phagocytes bind to various microbial carbohydrates such as those rich in mannose or fucose, and to N-acetylglucosamine (NAG). Human glycoproteins and glycolipids typically have terminal N-acetylglucosamine and sialic acid groups. C-type lectins found on the surface of phagocytes are mannose receptors (see Fig. 3). It is now thought that mannose receptors may be quite important in removing potentially harmful mannose-containing glycoproteins such as lysosomal hydrolases that are produced in increased amounts during inflammation.
Mannose-rich glycans are short carbohydrate chains with the sugar mannose or fructose as the terminal sugar. They are commonly found in microbial glycoproteins and glycolipids but are rare in those of humans. (Human glycoproteins and glycolipids typically have terminal N-acetylglucosamine and sialic acid groups.) C-type lectins, found on the surface of phagocytes, are endocytic pattern recognition receptors that bind to mannose-rich glycans in order to attach microbes to phagocytes. Mannose-binding lectin (MBL), also known as mannan-binding protein, is a soluble pattern recognition receptor in plasma and tissue fluid that binds to mannose-rich glycans on microbes in oder to activate the lectin complement pathway.
2. Dectin-1
Dectin-1 recognizes beta-glucans (polymers of gucose) commonly found in fungal cell walls.
3. Scavenger receptors
Scavenger receptors found on the surface of phagocytic cells bind to bacterial cell wall components such as LPS, peptidoglycan and teichoic acids (see Fig. 1). There are also scavenger receptors for certain components of other types of microorganisms, as well as for stressed, infected, or injured cells . Scavenger receptors include CD-36, CD-68, and SRB-1.
4. Opsonin receptors
Opsonins are soluble molecules produced as a part of the body's immune defenses that bind microbes to phagocytes. One portion of the opsonin binds to a PAMP on the microbial surface and another portion binds to a specific receptor on the phagocytic cell.
Acute phase proteins circulating in the plasma, such as:
mannose-binding lectin (also called mannose-binding protein) that binds to various microbial carbohydrates such as those rich in mannose or fucose, and to N-acetylglucosamine (NAG); and
C-reactive protein (CRP) that binds to phosphorylcholine portion of teichoic acids and lipopolysaccharides of bacterial and fungal cell walls. It also binds to the phosphocholine found on the surface of damaged or dead human cells.
Complement pathway proteins, such as C3b (see Fig. 4) and C4b recognize a variety of PAMPS.
Surfactant proteins in the alveoli of the lungs, such as SP-A and SP-D are opsonins.
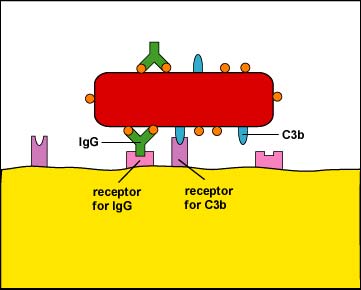
During adaptive immunity, the antibody molecule IgG can function as an opsonin (see Fig. 5).Fig. 4: Enhanced Attachment of Bacteria to Phagocytes by the Opsonin C3b
When body defense pathways known as the complement pathways are activated, one of the beneficial defense proteins made is called C3b. One portion of C3b binds to bacterial surface proteins and another portion binds to C3b receptors such as CD-1 on phagocytes. The process of enhanced attachment is called opsonization.
Fig. 5: Enhanced Attachment (Opsonization) of a Bacterium By Way Of the Antibody IgG
One of the functions of certain antibody molecules known as IgG is to stick antigens such as bacterial proteins and polysaccharides to phagocytes. The tips of the antibody, the Fab portion, have a shape that fits epitopes, portions of an antigen with a complementary shape. The stalkof the antibody is called the Fc portion and is able to bind to Fc receptors on phagocytes. Also, when body defense pathways known as the complement pathways are activated, one of the beneficial defense proteins made is called C3b. C3b binds by one end to bacterial surface proteins and by the other end to C3b receptors on phagocytes. The IgG and C3b are also known as opsonins and the process of enhanced attachment is also called opsonization.
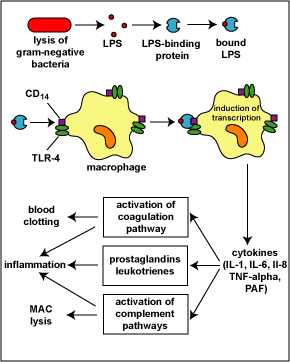
Fig. 7: Physiologic Action of Lipopolysaccharide (LPS) from the Gram-Negative Cell Wall
The lysis of gram-negative bacteria causes them to release lipopolysaccharide (LPS; endotoxin) from the outer membrane of their cell wall. The LPS binds to a LPS-binding protein circulating in the blood and this complex, in turn, binds to a receptor molecule (CD14) found on the surface of body defense cells called macrophages. This is thought to promote the ability of the toll-like receptor TLR-4 to respond to the LPS, triggering the macrophage to release various defense regulatory chemicals called cytokines, including IL-1, IL-6, IL-8, TNF-alpha, and PAF. The cytokines then bind to cytokine receptors on target cells and initiate inflammation and activate both the complement pathways and the coagulation pathway. (LPS, lipopolysaccharide; IL-1, interleukin-1; IL-6, interleukin-6; IL-8, interleukin-8, TNF-alpha, tumor necrosis factor-alpha; PAF, platelet-activating factor.) This will be discussed in greater detail under Bacterial Pathogenicity.
Another cell surface PRR is CD14. CD14 is found on monocytes, macrophages, and neutrophils and promotes the ability of TLR-4 to respond to LPS. LPS typically binds to LPS-binding protein in the plasma and tissue fluid. The LPS-binding protein promotes the binding of LPS to the CD14 receptors. At that point the LPS-binding protein comes off and the LPS-CD14 bind to TLR-4. Interaction of LPS and CD14 with TLR-4 leads to an elevated synthesis and secretion of inflammatory cytokines such as IL-1, IL-6, IL-8, TNF-alpha, and platelet-activating factor (PAF). These cytokines then bind to cytokine receptors on target cells and initiate inflammation and activate both the complement pathways and the coagulation pathway (see Fig. 7).
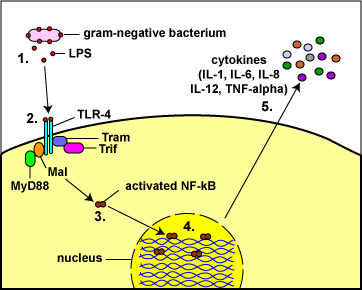
Fig. 8: Toll-Like Receptors Responding to Lipopolysaccharide (LPS)
from the Gram-Negative Cell Wall
(1) The lysis of Gram-negative bacteria causes them to release lipopolysaccharide (LPS; endotoxin) from the outer membrane of their cell wall.
(2) The LPS binds to a pair of TLR-4s on the macrophage.
(3&4) This enables regulatory molecules within the cell - Mal, MyD88, Tram, and Trif - to trigger reactions that activate a master regulator of inflammation called NF-kappa B. Activated NF-kappa B enters the cell's nucleus and switches on genes coding for cytokines such as:
a. Interleukin-1 (IL-1) and Tumor necrosis factor-alpha (TNF-alpha): enhance inflammatory responses;
b. Interleukin-8 (IL-8): aids in the ability of white blood cells to leave the blood vessels and enter the tissue; a chemoattractant for phagocytes;
c. Interleukin-6 (IL-6) promotes B-lymphocyte activity; and
d. Interleukin-12 (IL-12): promotes T-lymphocyte activity. (5)http://faculty.ccbcmd.edu/~gkaiser/SoftChalk%20BIOL%20230/Innate%20Immunity/PRRs/PRRs_print.html
Leaky Gut and Atopic Dermatitis: What Is the Connection?Tracing the skin as it rounds the lips and becomes the lining of the mouth and digestive tract, it seems reasonable that skin problems could continue on the inside as well. In fact, similar to the function of the skin, the lining of the intestines is tasked with keeping some things out (such as certain types of bacteria and larger molecules that may cause allergies) and allowing other things in (such as nutrients). An impairment of this function leads to increased permeability, sometimes called “leaky gut."
Certain triggers and stresses are thought to cause changes in the intestinal bacteria and disrupt the gut barrier, and this has been tied to many different diseases including inflammatory bowel disease, celiac disease, food intolerance, and small intestinal bacterial overgrowth. Interestingly, there are also non-gastrointestinal diseases on this list, including fibromyalgia, chronic fatigue syndrome, and, the focus of the current article, atopic dermatitis.跟踪皮肤,使其围绕嘴唇并成为口腔和消化道的衬里,因此皮肤问题也可能在内部继续存在是合理的。实际上,类似于皮肤的功能,肠壁的任务是阻止某些东西进入(例如某些类型的细菌和可能引起过敏的大分子),并允许其他东西进入(例如营养)。该功能的损害导致通透性增加,有时称为“渗漏性肠”。
人们认为某些触发因素和压力会引起肠道细菌的变化并破坏肠道屏障,而这与许多不同的疾病有关,包括炎症性肠病,腹腔疾病,食物不耐受和小肠细菌过度生长。有趣的是,该列表中还包含非胃肠道疾病,包括纤维肌痛,慢性疲劳综合症,以及本文的重点是特应性皮炎。
Abnormal Barrier in Atopic Dermatitis and Leaky Gut
In atopic dermatitis, the skin barrier is known to be abnormal: water escapes too easily leading to dry, itchy skin, and allergens, irritants, and bacteria can all enter, leading to an immune response and subsequent inflammation. Although there is some evidence to show that excluding foods from the diet in those without known allergy is not helpful for most, the implication of the gut in atopic dermatitis has been very difficult to shake, and many feel that diet is the “root cause.”
A study from the United Kingdom identified significantly increased intestinal permeability (leaky gut) in children with atopic dermatitis when compared to children without eczema, while another found a relationship between how leaky the gut was with how severe the eczema was--a very compelling finding, to be sure!
If leaky gut is indeed connected with atopic dermatitis, can it be treated? The answer seems to be “yes,” but with qualifications.特应性皮炎和肠漏的异常屏障
在特应性皮炎中,已知皮肤屏障异常:水很容易逸出,导致皮肤干燥,发痒,并且过敏原,刺激物和细菌都可以进入,导致免疫反应和随后的炎症。尽管有证据表明,对那些没有过敏症的人从饮食中排除食物对大多数人无济于事,但肠胃对特应性皮炎的影响却很难动摇,许多人认为饮食是“根本原因”。 ”
英国的一项研究发现,与没有湿疹的儿童相比,特应性皮炎患儿的肠道通透性(肠道渗漏)显着增加,而另一项研究发现,肠道渗漏与湿疹的严重程度之间存在关联-这是一个非常令人信服的发现, 为了确定!
如果肠道渗漏确实与特应性皮炎有关,可以治疗吗?答案似乎是“是”,但有条件。
Neurotherapeutics
January 2018, Volume 15, Issue 1, pp 75–91 | Cite as
Diet, Gut Microbiota, and Vitamins D + A in Multiple Sclerosis
1.Department of Sciences University of Basilicata Potenza Italy
Abstract
Central to the understanding of the relationships between diet, gut microbiota, and vitamins D and A in multiple sclerosis is low-grade inflammation, which is involved in all chronic inflammatory diseases and is influenced by each of the above effectors. We show that food components have either proinflammatory or anti-inflammatory effects and influence both the human metabolism (the “metabolome”) and the composition of gut microbiota. Hypercaloric, high-animal-fat Western diets favor anabolism and change gut microbiota composition towards dysbiosis. Subsequent intestinal inflammation leads to leakage of the gut barrier, disruption of the blood–brain barrier, and neuroinflammation. Conversely, a vegetarian diet, rich in fiber, is coherent with gut eubiosis and a healthy condition. Vitamin D levels, mainly insufficient in a persistent low-grade inflammatory status, can be restored to optimal values only by administration of high amounts of cholecalciferol. At its optimal values (>30 ng/ml), vitamin D requires vitamin A for the binding to the vitamin D receptor and exert its anti-inflammatory action. Both vitamins must be supplied to the subjects lacking vitamin D. We conclude that nutrients, including the nondigestible dietary fibers, have a leading role in tackling the low-grade inflammation associated with chronic inflammatory diseases. Their action is mediated by gut microbiota and any microbial change induced by diet modifies host–microbe interactions in a consequent way, to improve the disease or worsen it.
Keywords
Diet Gut Microbiota Vitamin D Multiple Sclerosis NeuroinflammationDiet, Gut Microbiota, and Vitamins D + A in Multiple Sclerosis | SpringerLink
https://link.springer.com/article/10.1007%2Fs13311-017-0581-4
Published: 03 May 2019
The combination of sport and sport-specific diet is associated with characteristics of gut microbiota: an observational study
Lae-Guen Jang, Geunhoon Choi, Sung-Woo Kim, Byung-Yong Kim, Sunghee Lee & Hyon Park
Journal of the International Society of Sports Nutrition volume 16, Article number: 21 (2019)
Abstract
Background
Recently, gut microbiota have been studied extensively for health promotion, disease prevention, disease treatment, and exercise performance. It is recommended that athletes avoid dietary fiber and resistant starch to promote gastric emptying and reduce gastrointestinal distress during exercise, but this diet may reduce microbial diversity and compromise the health of the athlete’s gut microbiota.
Objective
This study compared fecal microbiota characteristics using high-throughput sequencing among healthy sedentary men (as controls), bodybuilders, and distance runners, as well as the relationships between microbiota characteristics, body composition, and nutritional status.
Methods
Body composition was measured using DXA, and physical activity level was assessed using IPAQ. Dietary intake was analyzed with the computerized nutritional evaluation program. The DNA of fecal samples was extracted and it was sequenced for the analysis of gut microbial diversity through bioinformatics cloud platform.
Results
We showed that exercise type was associated with athlete diet patterns (bodybuilders: high protein, high fat, low carbohydrate, and low dietary fiber diet; distance runners: low carbohydrate and low dietary fiber diet). However, athlete type did not differ in regard to gut microbiota alpha and beta diversity. Athlete type was significantly associated with the relative abundance of gut microbiota at the genus and species level: Faecalibacterium, Sutterella, Clostridium, Haemophilus, and Eisenbergiella were the highest (p < 0.05) in bodybuilders, while Bifidobacterium and Parasutterella were the lowest (p < 0.05). At the species level, intestinal beneficial bacteria widely used as probiotics (Bifidobacterium adolescentis group, Bifidobacterium longum group, Lactobacillus sakei group) and those producing short chain fatty acids (Blautia wexlerae, Eubacterium hallii) were the lowest in bodybuilders and the highest in controls. In addition, aerobic or resistance exercise training with an unbalanced intake of macronutrients and low intake of dietary fiber led to similar diversity of gut microbiota. Specifically, daily protein intake was negatively correlated with operation taxonomic unit (r = − 0.53, p < 0.05), ACE (r = − 0.51, p < 0.05), and Shannon index (r = − 0.64, p < 0.01) in distance runners..
Conclusion
Results suggest that high-protein diets may have a negative impact on gut microbiota diversity for athletes, while athletes in resistance sports that carry out the high protein low carbohydrates diet demonstrate a decrease in short chain fatty acid-producing commensal bacteria.The combination of sport and sport-specific diet is associated with characteristics of gut microbiota: an observational study | Journal of the International Society of Sports Nutrition | Full Text
https://jissn.biomedcentral.com/articles/10.1186/s12970-019-0290-y
J Cell Biochem. 2018 Dec;119(12):9997-10004. doi: 10.1002/jcb.27329. Epub 2018 Aug 26.
LPS promotes the expression of PD-L1 in gastric cancer cells through NF-κB activation.
Li H1, Xia JQ1, Zhu FS1, Xi ZH1, Pan CY1, Gu LM1, Tian YZ1.
Author information
1
Department of Gastroenterology, Affiliated Hospital of Integrated Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing, Jiangsu, China.
Abstract
Gastric cancers are a group of highly aggressive malignancies with a huge disease burden worldwide. Gastric infections, such as helicobacter pylori, can induce the occurrence of gastric cancers. However, the role of gastric infection in gastric cancer development is unclear. Programmed death-ligand 1 (PD-L1, B7-H1) is a member of the B7 family of cell surface ligands, which binds the PD-1 transmembrane receptor and inhibits T-cell activation within cancer tissues. It has been reported that the expression of PD-L1 is inversely related to the prognosis of patients with gastric cancers. Therefore, the regulation of PD-L1 expression in gastric cancers needs to be studied. In the current study, we explored the possible effects of lipopolysaccharide (LPS) on PD-L1 expression in gastric cancer cells. We observed that LPS stimulation could markedly increase PD-L1 expression in gastric cancer cells. Furthermore, we found that nuclear factor-κB (NF-κB) activation was involved in PD-L1 expression in gastric cancer cells exposed to LPS stimulation through p65-binding to the PD-L1 promoter. Taken together, these data indicate that gastric infection might promote the development of gastric cancers thought the LPS-NF-κB-PD-L1 axis.
J细胞生物化学。2018年12月;119(12):9997-10004。doi: 10.1002 / jcb.27329。Epub 2018 8月26日。
LPS促进PD-L1的表达在胃癌细胞通过NF-κB激活
Li H1, Xia JQ1, Zhu FS1, Xi ZH1, Pan CY1, Gu LM1, Tian YZ1。
作者信息
1 南京中医药大学中西医结合医院消化内科,江苏南京。
摘要
胃癌是一组恶性程度极高的恶性肿瘤,在世界范围内具有巨大的疾病负担。胃感染,如幽门螺杆菌感染,可引起胃癌的发生。然而,胃感染在胃癌发生发展中的作用尚不明确。程序性死亡配体1 (PD-L1, B7- h1)是细胞表面配体B7家族的成员之一,它与PD-1跨膜受体结合,抑制癌细胞组织内T细胞的活化。有报道称PD-L1的表达与胃癌患者的预后呈负相关。因此,PD-L1在胃癌中的表达调控需要进一步研究。在本研究中,我们探讨了脂多糖(LPS)对胃癌细胞中PD-L1表达的可能影响。我们观察到LPS刺激可以显著增加胃癌细胞中PD-L1的表达。此外,我们发现核factor-κB (NF-κB)激活参与PD-L1表达在胃癌细胞暴露于LPS刺激通过p65-binding PD-L1启动子。综上所述,这些数据表明胃感染可能促进胃癌症的发展认为LPS-NF-κB-PD-L1轴。
©2018威利期刊有限公司
© 2018 Wiley Periodicals, Inc.LPS promotes the expression of PD-L1 in gastric cancer cells through NF-κB activation. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/30145830
Dietary Emulsifier–Induced Low-Grade Inflammation Promotes Colon Carcinogenesis
膳食乳化剂诱导的轻度炎症促进结肠癌发生
埃米莉·维恩努瓦(Emilie Viennois),迪迪埃·梅林(Didier Merlin),安德鲁·T·古维兹(Andrew T.
DOI:10.1158 / 0008-5472.CAN-16-1359发布于2017年1月
文章图形与数据信息与度量PDF
抽象
炎症性肠病(IBD)赋予结直肠癌发展的风险增加,产生了术语“结肠炎相关癌”和炎症促进结肠肿瘤发生的概念。比IBD更常见的疾病是低度炎症,这与许多大肠癌病例中都存在的肠道微生物群组成改变和代谢综合征有关。最近的发现表明,食用乳化剂可促进肠道低度炎症,而食用乳化剂是加工食品中普遍存在的成分,可改变肠道菌群的组成。在这里,我们在结肠炎诱发的大肠癌的临床前模型中证明,定期食用饮食乳化剂,羧甲基纤维素或聚山梨酯80会加剧肿瘤的发展。增强的肿瘤发展与改变的微生物组元基因组有关,其特征在于脂多糖和鞭毛蛋白水平升高。我们发现,乳化剂在微生物组中引起的改变是必要的,并且足以驱动主要的增殖和凋亡信号通路的改变,这些通路被认为可以控制肿瘤的发展。总体而言,我们的发现支持这样的概念,即引起轻度肠道炎症的宿主-微生物群相互作用的扰动可促进结肠癌的发生。癌症研究; 77(1); 27-40。 ©2016 AACR。Dietary Emulsifier–Induced Low-Grade Inflammation Promotes Colon Carcinogenesis | Cancer Research
https://cancerres.aacrjournals.org/content/77/1/27
高脂饮食通过破坏肠细胞膜完整性来加速肠肿瘤发生
癌症预防杂志2016; 21:95-103
在线发布于2016年6月30日
©2016韩国癌症预防学会。
背景:
过多的能量供应会在包括肠上皮在内的各种组织中引起慢性低度炎症,并伴有氧化应激。这项研究的目的是调查高脂饮食(HFD)对ApcMin / +小鼠肠道细胞膜完整性和肠道肿瘤发生的影响。
方法:
用正常饮食(ND)或HFD喂养小鼠12周。计算肠道肿瘤的数量,并确定内毒素血症,氧化应激和炎症的生物标志物。通过异硫氰酸荧光素(FITC)-葡聚糖渗透和膜间隙连接蛋白表达来测量肠完整性的变化。
结果:
与ND组相比,HFD组的肿瘤数目显着更高(P <0.05)。与ND组相比,HFD组的血液总抗氧化能力较低,而氧化损伤的标志物结肠8-羟基-2'-脱氧鸟苷水平较高(P <0.05)。 HFD组FITC-右旋糖酐的渗透率显着增加(P <0.05),而HFD组膜间隙连接蛋白包括zonula occludens-1,claudin-1和occludin的表达较低。High-fat Diet Accelerates Intestinal Tumorigenesis Through Disrupting Intestinal Cell Membrane Integrity
Journal of Cancer Prevention 2016;21:95-103
Published online June 30, 2016
© 2016 Korean Society of Cancer Prevention.
Background:
Excess energy supply induces chronic low-grade inflammation in association with oxidative stress in various tissues including intestinal epithelium. The objective of this study was to investigate the effect of high-fat diet (HFD) on intestinal cell membrane integrity and intestinal tumorigenesis in ApcMin/+ mice.
Methods:
Mice were fed with either normal diet (ND) or HFD for 12 weeks. The number of intestinal tumors were counted and biomarkers of endotoxemia, oxidative stress, and inflammation were determined. Changes in intestinal integrity was measured by fluorescein isothiocyanate (FITC)-dextran penetration and membrane gap junction protein expression.
Results:
HFD group had significantly higher number of tumors compared to ND group (P < 0.05). Blood total antioxidant capacity was lower in HFD group, while colonic 8-hydroxy-2′-deoxyguanosine level, a marker of oxidative damage, was higher in HFD group compared to that of ND group (P < 0.05). The penetration of FITC-dextran was substantially increased in HFD group (P < 0.05) while the expressions of membrane gap junction proteins including zonula occludens-1, claudin-1, and occludin were lower in HFDHigh-fat Diet Accelerates Intestinal Tumorigenesis Through Disrupting Intestinal Cell Membrane Integrity
http://www.jcpjournal.org/journal/view.html?doi=10.15430/JCP.2016.21.2.95
Article
Published: 07 May 2019
Lipopolysaccharide induces the differentiation of hepatic progenitor cells into myofibroblasts constitutes the hepatocarcinogenesis-associated microenvironment
Wen-ting Liu, Ying-ying Jing, Lu Gao, Rong Li, Xue Yang, Xiao-rong Pan, Yang Yang, Yan Meng, Xiao-juan Hou, Qiu-dong Zhao, Zhi-peng Han & Li-xin Wei
Cell Death & Differentiation volume 27, pages85–101(2020)Cite this article
Abstract
Hepatocellular carcinoma (HCC) generally occurs in the presence of chronic liver injury, often as a sequela of liver fibrosis. Hepatic progenitor cells (HPCs) are known to be capable of forming both hepatocytes and cholangiocytes in chronic liver injury, which are also considered a source of myofibroblasts and tumor-initiating cells, under carcinogenic circumstances. However, the underlying mechanisms that activate HPCs to give rise to HCC are still unclear. In current study, the correlation between HPCs activation and liver fibrosis and carcinogenesis was investigated in rats and human specimens. We analyzed the role of HPCs in tumorigenesis, by transplanting exogenous HPCs in a diethylnitrosamine-induced rat HCC model. Our data indicated that HPC activation correlated with hepatic fibrosis and hepatocarcinogenesis. We further found that exogenous HPC infusion promoted liver fibrosis and hepatocarcinogenesis, while lipopolysaccharides (LPS) played an important role in this process. However, results of our study indicated that LPS did not induce HPCs to form tumor in nude mice directly. Rather, LPS induced myofibroblast-like morphology in HPCs, which enhanced the tumorigenic potential of HPCs. Further experiments showed that LPS/Toll-like receptor 4 (TLR4) signaling mediated the differentiation of HPCs into myofibroblasts and enhanced the production of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), which led to the aberrant expression of Ras and p53 signaling pathways in HPCs, and finally, promoted the proliferation and malignant transformation of HPCs, by long non-coding RNA regulation. Besides, examination of HCC clinical samples demonstrated that IL-6 and TNF-α production correlated with HPC activation, hepatic fibrosis, and HCC recurrence. Our study indicates that both myofibroblasts and tumor cells are derived from HPCs. HPC-derived myofibroblasts create tumor microenvironment and contribute to the proliferation and malignant transformation of HPCs. Furthermore, LPS present in the chronic liver inflammation microenvironment might play an important role in hepatocarcinogenesis, by regulating the plastic potential of HPCs.Lipopolysaccharide induces the differentiation of hepatic progenitor cells into myofibroblasts constitutes the hepatocarcinogenesis-associated microenvironment | Cell Death & Differentiation
https://www.nature.com/articles/s41418-019-0340-7
Ferrichrome is a cyclic hexa- peptide that forms a complex with iron atoms. It is a siderophore composed of three glycine and three modified ornithine residues with hydroxamate groups [-N(OH)C(=O)C-]. The 6 oxygen atoms from the three hydroxamate groups bind Fe(III) in near perfect octahedral coordination. Ferrichrome was first isolated in 1952, has been found to be produced by fungi of the genera Aspergillus , Ustilago , and Penicillium . Ferrichrome - Wikipedia en.wikipedia.org/wiki/Ferrichrome
Probiotic-derived ferrichrome inhibits colon cancer progression via JNK-mediated apoptosis
1Division of Gastroenterology and Hematology/Oncology, Department of Medicine, Asahikawa Medical University, Asahikawa 078-8510, Japan
Previous reports have suggested that some probiotics inhibit tumorigenesis and cancer progression. However, the molecules involved have not yet been identified. Here, we show that the culture supernatant of Lactobacillus casei ATCC334 has a strong tumour-suppressive effect on colon cancer cells. Using mass spectrometry, we identify ferrichrome as a tumour-suppressive molecule produced by Lactobacillus casei ATCC334. The tumour-suppressive effect of ferrichrome is greater than that of cisplatin and 5-fluorouracil, and ferrichrome has less of an effect on non-cancerous intestinal cells than either of those agents. A transcriptome analysis reveals that ferrichrome treatment induces apoptosis, which is mediated by the activation of c-jun N-terminal kinase (JNK). Western blotting indicates that the induction of apoptosis by ferrichrome is reduced by the inhibition of the JNK signalling pathway. This we demonstrate that probiotic-derived ferrichrome exerts a tumour-suppressive effect via the JNK signalling pathway.
Probiotic-derived ferrichrome inhibits colon cancer progression via JNK-mediated apoptosis
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4987524/
Lipopolysaccharide induces inflammation and facilitates lung metastasis in a breast cancer model via the prostaglandin E2-EP2 pathway
Inflammation is a potent promoter of tumor metastasis. The aim of the present study was to explore the function of systemic inflammation in the formation of lung metastasis of breast cancer cells in a mouse model. BALB/c mice were injected intraperitoneally with lipopolysaccharide (LPS) in order to establish an inflammatory animal model and 4T1 murine breast cancer cells were injected through the tail vein to induce lung metastasis. The levels of proinflammatory cytokines were evaluated by ELISA. Metastases on the surface of the lungs were counted and histologically analyzed by hematoxylin and eosin staining. Angiogenesis in the lungs was examined by CD31 immunofluorescence. Mouse pulmonary endothelial cells (MPVECs) were isolated and used to assay endothelial tube formation and determine the protein expression levels of vascular endothelial growth factor (VEGF) in vitro. Serum levels of VEGF and prostaglandin E2 (PGE2), the number and size of metastatic lesions, and the expression levels of cyclooxygenase‑2 were significantly greater in the lungs of LPS‑treated mice, as compared with those in control mice threated with phosphate‑buffered saline. Blood vessel density was also markedly increased in the LPS‑treated mice. These increases were reversed by treatment with celecoxib. In vitro, the protein expression levels of VEGF produced by the PGE2‑treated cells were significantly increased in a concentration‑dependent manner. In addition, the production of VEGF was increased in response to treatment with the PGE2 receptor (EP2) agonist ONO‑AE1‑259‑01; however, this increase was abrogated by treatment with AH6809, an EP2 receptor antagonist. Treatment with PGE2 or VEGF alone promoted the tube formation of MPVECs and this effect was reversed by treatment with celecoxib. These results demonstrated that PGE2 may regulate the release of VEGF by MPVECs through the EP2 receptor pathway and thereby promoted pulmonary angiogenesis and breast cancer metastasis in a mouse model.
Introduction
Lipopolysaccharide induces inflammation and facilitates lung metastasis in a breast cancer model via the prostaglandin E2-EP2 pathway https://www.spandidos-publications.com/10.3892/mmr.2015.3258
Infection. 2018 Dec;46(6):751-760. doi: 10.1007/s15010-018-1178-5. Epub 2018 Jul 12.
Gut-origin sepsis in the critically ill patient: pathophysiology and treatment.
Assimakopoulos SF1, Triantos C2, Thomopoulos K2, Fligou F3, Maroulis I4, Marangos M5, Gogos CA5.
Author information 1Department of Internal Medicine, Division of Infectious Diseases, University of Patras Medical School, 26504, Patras, Greece. sassim@upatras.gr.2Department of Internal Medicine, Division of Gastroenterology, University of Patras Medical School, 26504, Patras, Greece.3Department of Anesthesiology and Critical Care Medicine, University of Patras Medical School, 26504, Patras, Greece.4Department of Surgery, University of Patras Medical School, 26504, Patras, Greece.5Department of Internal Medicine, Division of Infectious Diseases, University of Patras Medical School, 26504, Patras, Greece.
Abstract
INTRODUCTION: Gut permeability is increased in critically ill patients, and associated with the development of the systemic inflammatory response syndrome and multiple organ dysfunction syndrome (MODS). The pathogenetic link(s) and potential therapies are an area of intense research over the last decades.
METHODS: We thoroughly reviewed the literature on gut-origin sepsis and MODS in critically ill patients, with emphasis on the implicated pathophysiological mechanisms and therapeutic interventions.
FINDINGS:
Intestinal barrier failure leading to systemic bacterial translocation associated with MODS was the predominant pathophysiological theory for several years. However, clinical studies with critically ill patients failed to provide the evidence of systemic spread of gut-derived bacteria and/or their products as a cause of MODS. Newer experimental data highlight the role of the mesenteric lymph as a carrier of gut-derived danger-associated molecular patterns (DAMPs) to the lung and the systemic circulation. These substances are recognized by pattern recognition receptor-bearing cells in diverse tissues and promote proinflammatory pathways and the development MODS. Therefore, the gut becomes a pivotal proinflammatory organ, driving the systemic inflammatory response through DAMPs release in mesenteric lymph, without the need for systemic bacterial translocation.
CONCLUSIONS: There is an emerging need for application of sensitive non-invasive and easily measured biomarkers of early intestinal injury (e.g., citrulline, intestinal fatty acid protein, and zonulin) in our everyday clinical practice, guiding the early pharmacological intervention in critically ill patients to restore or prevent intestinal injury and improve their outcomes.
KEYWORDS: Bacterial translocation; Danger-associated molecular patterns; Gut-lymph hypothesis; Gut-origin sepsis; ICU; Intestinal barrier; Intestinal permeability
Gut-origin sepsis in the critically ill patient: pathophysiology and treatment. - PubMed - NCBI https://www.ncbi.nlm.nih.gov/pubmed/30003491
Crit Care Clin. 2016 Apr;32(2):203-12. doi: 10.1016/j.ccc.2015.11.004. Epub 2016 Feb 4.
The Gut as the Motor of Multiple Organ Dysfunction in Critical Illness.
Klingensmith NJ1, Coopersmith CM2.
Author information
1
Department of Surgery, Emory Critical Care Center, Emory University School of Medicine, Atlanta, GA, USA.
2
Department of Surgery, Emory Critical Care Center, Emory University School of Medicine, Atlanta, GA, USA. Electronic address: cmcoop3@emory.edu.
Abstract
All elements of the gut - the epithelium, the immune system, and the microbiome - are impacted by critical illness and can, in turn, propagate a pathologic host response leading to multiple organ dysfunction syndrome. Preclinical studies have demonstrated that this can occur by release of toxic gut-derived substances into the mesenteric lymph where they can cause distant damage. Further, intestinal integrity is compromised in critical illness with increases in apoptosis and permeability. There is also increasing recognition that microbes alter their behavior and can become virulent based upon host environmental cues. Gut failure is common in critically ill patients; however, therapeutics targeting the gut have proven to be challenging to implement at the bedside. Numerous strategies to manipulate the microbiome have recently been used with varying success in the ICU.The Gut as the Motor of Multiple Organ Dysfunction in Critical Illness. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/27016162
Autophagy
Taylor & Francis
Lipopolysaccharide mediates hepatic stellate cell activation by regulating autophagy and retinoic acid signaling
Ming Chen, Jiaxing Liu, [...], and Wenhua Ling
aDepartment of Nutrition, School of Public Health, Sun Yat-Sen University, Guangzhou, Guangdong, People's Republic of China
bGuangdong Provincial Key Laboratory of Food, Nutrition and Health, Guangzhou, Guangdong, China
ABSTRACT
Bacterial translocation and lipopolysaccharide (LPS) leakage occur at a very early stage of liver fibrosis in animal models. We studied the role of LPS in hepatic stellate cell (HSC) activation and the underlying mechanisms in vitro and in vivo. Herein, we demonstrated that LPS treatment led to a dramatic increase in autophagosome formation and autophagic flux in LX-2 cells and HSCs, which was mediated through the AKT-MTOR and AMPK-ULK1 pathway. LPS significantly decreased the lipid content, including the lipid droplet (LD) number and lipid staining area in HSCs; pretreatment with macroautophagy/autophagy inhibitors or silencing ATG5 attenuated this decrease. Furthermore, lipophagy was induced by LPS through the autophagy-lysosomal pathway in LX-2 cells and HSCs. Additionally, LPS-induced autophagy further reduced retinoic acid (RA) signaling, as demonstrated by a decrease in the intracellular RA level and Rar target genes, resulting in the downregulation of Bambi and promoting the sensitization of the HSC's fibrosis response to TGFB. Compared with CCl4 injection alone, CCl4 plus LPS injection exaggerated liver fibrosis in mice, as demonstrated by increased Col1a1 (collagen, type I, α 1), Acta2, Tgfb and Timp1 mRNA expression, ACTA2/α-SMA and COL1A1 protein expression, and Sirius Red staining area, which could be attenuated by injection of an autophagy inhibitor. LPS also reduced lipid content in HSCs in vivo, with this change being attenuated by chloroquine (CQ) administration. In conclusion, LPS-induced autophagy resulted in LD loss, RA signaling dysfunction, and downregulation of the TGFB pseudoreceptor Bambi, thus sensitizing HSCs to TGFB signaling.
KEYWORDS: autophagy, BAMBI, hepatic stellate cell, liver fibrosis, LPS, retinoic acid signaling, TGFB
INTRODUCTION
Liver fibrosis represents an adaptive response to repeated chronic liver injuries, which are primarily caused by chronic viral hepatitis and steatohepatitis associated with either alcohol consumption or obesity.1, 2 Studies using animal models of liver fibrosis have demonstrated that myofibroblasts, which are not present in normal liver tissue, play a crucial role in hepatic fibrogenesis. Myofibroblasts are derived from 3 cellular sources, liver resident hepatic stellate cells (HSCs), portal fibroblasts and bone marrow-derived collagen-producing cells, and the composition of myofibroblasts varies depending on the etiology of liver fibrosis.3 However, activated HSCs are the predominant type of myofibroblasts observed during the progression of liver fibrosis.4 Quiescent HSCs, which are located in the space of Disse and store retinoids in lipid droplets (LDs), transform into an activated phenotype characterized by the induction of fibrotic markers and depletion of LDs. However, the mechanisms underlying HSC activation remain unclear. Therefore, elucidating these HSC activation events could promote new strategies for preventing and treating liver fibrosis.
Autophagy is an evolutionarily conserved process in which cytoplasmic constituents, including damaged and dysfunctional proteins and organelles, are delivered to lysosomes for degradation and recycling of the breakdown products.5 Macroautophagy (hereafter referred to as autophagy) involves the formation of double-membraned vesicles called autophagosomes and the subsequent fusion of autophagosomes with lysosomes for cargo recycling to maintain cellular homeostasis.6 Defects in autophagy have been closely associated with many human diseases, including cancer, infection and neurodegeneration.7-9 Recent studies have demonstrated that functional autophagy is involved in lipid clearance in hepatocytes, a process termed lipophagy.10-12 Previous studies have shown that autophagy plays a critical role in lipid clearance and fibrotic activity,13-15 and this effect is associated with the liberation of free fatty acids from LDs and subsequent mitochondrial β-oxidation, which provides energy to support HSC activation.16Bacterial lipopolysaccharide (LPS), a cell wall component of gram-negative bacteria that is among the strongest known inducers of inflammation, has been found to be associated with hepatic fibrogenesis through direct interactions with HSCs.17 During chronic liver injury caused by CCl4 injection or bile duct ligation (BDL), the plasma concentration of LPS is significantly elevated even at a very early stage due to changes in the intestinal mucosal permeability and increased bacterial translocation.17, 18 Consistent with these reports, one recent study observed that the transplantation of Gram-negative bacteria affects hepatic fibrogenesis after BDL.19 However, the molecular mechanism underlying the effects of LPS on HSC activation is poorly understood. Recent studies have shown that LPS induces autophagy in macrophages, linking 2 ancient processes, autophagy and innate immunity.20-22 Here, we hypothesized that the LPS regulates HSC activation through autophagy and retinoic acid signaling.
Results
LPS treatment induced autophagic markers in LX-2 cells
LPS exposure promoted autophagic flux in LX-2 cells and HSCs
The AKT-MTOR signaling pathway mediated LPS-induced autophagy
The AKT-MTOR cascade is considered a major negative regulator of autophagy in multiple types of cell.10, 23,24 Therefore, we examined whether this signaling pathway was also involved in LPS-induced autophagy. As shown in Fig. 2, LPS treatment led to significantly decreased phosphorylation of AKT at Thr308 and MTOR at Ser2448, and increased phosphorylation of AMPK at Thr172and ATG5 expression.
LPS stimulated HSC lipophagy
LPS mediated the downregulation of Bambi through autophagy and the subsequent promotion of the HSC fibrotic response to TGFB signaling
LPS-induced RA signaling dysfunction inhibited Bambi expression and promoted HSC activation
Autophagy is involved in the LPS-mediated augmentation of the fibrotic response in CCl4-treated mice
LPS induced autophagy and decreased RA signaling in HSCs in vivo
In summary, the present study demonstrates that LPS exposure promotes HSC fibrosis through increased autophagy activity and dysfunctional RA signaling. This novel mechanism underlying the LPS-induced fibrotic response of HSCs is associated with LDs loss and downregulation of the TGFB pseudoreceptor BAMBI.
Lipopolysaccharide mediates hepatic stellate cell activation by regulating autophagy and retinoic acid signaling
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5788469/#__ffn_sectitle
Probiotics for the airways: Potential to improve epithelial and immune homeostasis
K. Martens B. Pugin I. De Boeck I. Spacova B. Steelant S. F. Seys S. Lebeer P. W. Hellings
First published: 05 June 2018 https://doi.org/10.1111/all.13495 Citations: 12
The authors are supported by grants from the Belgian Federal Government (IUAP P7/30), the Institute for Science and Technology Flanders (IWT) (TBM project 130260), the research council of the KU Leuven (GOA 2009/07 and 14/011), and IWT‐SBO grant (ProCure, 150052).
Abstract
Probiotics are live microorganisms that, when administered in adequate amounts, confer health benefit on the host. The therapeutic effects of probiotics have been mostly studied in the gastrointestinal tract, but recent evidence points toward the potential of these bacteria to prevent and/or treat chronic airway diseases. In this review, possible mechanisms of action of probiotics in the airways are described, with a particular focus on their capacity to modulate the epithelial barrier function and their mode of interaction with the immune system. Indeed, probiotic bacteria, mostly lactobacilli, can promote the expression and regulation of tight junctions and adherence junctions, resulting in the restoration of a defective epithelial barrier. These bacteria interact with the epithelial barrier and immune cells through pattern recognition receptors, such as Toll‐like receptors, which upon activation can stimulate or suppress various immune responses. Finally, the clinical potential of probiotics to treat inflammatory diseases of the upper and lower respiratory tract, and the difference between their mode of application (eg, oral or nasal) are discussed here.
气道益生菌:改善上皮和免疫稳态的潜力
K.Martens B.Pugin I.De Boeck I.Spacova B.Steantant S.F.Seys S.Lebeer P.W.Hellings
首次发布:2018年6月5日https://doi.org/10.1111/all.13495引用次数:12
作者得到比利时联邦政府(IUAP P7 / 30),法兰德斯科学技术研究院(TBW项目130260),鲁汶大学研究理事会(GOA 2009/07和14/011)的资助。 )和IWT‐SBO拨款(ProCure,150052)。
抽象
益生菌是活的微生物,当以足够的量使用时,可以赋予宿主健康。益生菌的治疗作用主要在胃肠道中进行了研究,但最近的证据表明这些细菌具有预防和/或治疗慢性气道疾病的潜力。在这篇综述中,描述了益生菌在气道中的可能作用机理,特别关注其调节上皮屏障功能的能力及其与免疫系统相互作用的方式。确实,益生菌细菌(主要是乳杆菌)可以促进紧密连接和粘附连接的表达和调节,从而恢复有缺陷的上皮屏障。这些细菌通过模式识别受体(例如Toll样受体)与上皮屏障和免疫细胞相互作用,激活后可以刺激或抑制各种免疫反应。最后,本文讨论了益生菌治疗上呼吸道和下呼吸道炎性疾病的临床潜力,以及它们的应用方式(例如口服或经鼻)之间的差异。
Abbreviations
AHR
Airway hyper‐reactivity
AJ
Adherence junction
AR
Allergic rhinitis
BALF
Bronchoalveolar lavage fluid
CRS
Chronic rhinosinusitis
DCs
Dendritic cells
IEC
Intestinal epithelial cell
IL
Interleukin
LAB
Lactic acid bacteria
LPS
Lipopolysaccharides
MAMPs
Microorganism‐associated molecular patterns
NGS
Next‐generation sequencing
NLRs
NOD‐like receptors
OVA
Ovalbumin
PRRs
Pattern recognition receptors
TGF‐β
Transforming growth factor β
TJ
Tight junction
TLRs
Toll‐like receptors
TNF‐α
Tumor necrosis factor‐α
Tregs
Regulatory T cells
URT
Upper respiratory tract
ZO
Zonula occludens
1 INTRODUCTION
The human body is colonized by approximately 100 trillion microbes, collectively known as the microbiota, which play an essential role in our well‐being.1 Thanks to the development of advanced culture‐independent tools, such as next‐generation sequencing, insights have been obtained in possible associations of the microbiota composition with disease.2, 3 Together with additional experimental and clinical data on various roles of the microbiota, it is now widely accepted that the microbiota provides the host with a wide array of health effects. These health effects include (i) exclusion or inhibitions of pathogens,4, 5 (ii) enhancement of epithelial barrier function by the modulation of signaling pathways,6, 7 and (iii) strain‐specific modulation of local and systemic host immune responses (Figure 1).8
Figure 1
Open in figure viewerPowerPoint
Relationship between microbiota diversity, epithelial Integrity, and inflammation in disease state
The clinical potential of probiotics has gained interest as a novel oral and/or nasal treatment option for chronic airway diseases. Probiotics interact, via their microorganism‐associated molecular patterns (MAMPs), with pattern recognition receptors (PRRs) on epithelial cells. This interaction can modulate tight junctions (TJs) and adherence junctions (AJs), which results in the restoration of a defective epithelial barrier. Probiotics are able to modulate host immune responses via interactions with dendritic cells (DCs.), which on their turn promote regulatory T cells (Tregs) and reduce T helper 1 (Th1) and T helper 2 (Th2) cells.
The involvement of the microbiota in the development or exacerbation of chronic noninfectious diseases is also increasingly considered.9 An imbalance of the microbiota composition, also known as dysbiosis, has been shown in various studies to negatively impact the health status.10 Three subtypes of dysbiosis have been identified as follows: (i) loss of beneficial microbial agents, (ii) expansion of potentially harmful microorganisms, and (iii) loss of overall microbial diversity.10 Microbial dysbiosis has been identified in different chronic inflammatory diseases, including chronic rhinosinusitis (CRS),11, 12 asthma,13, 14 Crohn's disease,15 and ulcerative colitis.16 Interestingly, in these chronic diseases, a disturbed permeability and function of the epithelial barrier have also been demonstrated.17-19 It is, however, not known whether microbiota dysbiosis could be the cause or simply a consequence of the associated epithelial barrier disruption.
When looking specifically at the airways, various microorganisms such as, Staphylococcus aureus,20 Streptococcus pneumoniae, and Haemophilus influenzae21 could induce pathogenic effects on the host, at least in part by increasing the epithelial barrier permeability in both the upper and lower airways. On the contrary, other microorganisms—both endogenous and exogenous—can also provide beneficial effects22 (Figure 2). These beneficial bacteria are called probiotics, defined as live microorganisms that, upon administration in adequate amounts, can provide health benefits to the host.23 Traditionally, Lactobacillus and Bifidobacterium strains have been used as probiotics, mainly to reduce gastrointestinal tract discomfort. Other bacterial taxa have probiotic potentials, such as the Streptococcus taxa24 or Dolosigranulum which is associated with respiratory health in different microbiome studies,25, 26 but these studies are limited so that this review will mainly focus on the Lactobacillus strains.
image
Figure 2
Open in figure viewerPowerPoint
Interaction of probiotics with the epithelial barrier and the immune system. Probiotics induce their health‐promoting capacities via different mechanisms. These bacteria are able to enhance epithelial barrier function via modulation of the intercellular junctions (TJs, AJs, and desmosomes) or via interaction with the different PRRs, present on the epithelial barrier. These PRRs (ie, TLRs, CLRs, NLRs) recognize the MAMPs, such as LTA, CPS, and LPS, present on the probiotic cell surface. Probiotics are also able to modulate the local and systemic immune responses. They have the ability to interact with DCs, present between the epithelial cells or in the submucosal region. This interaction can result in the activation of Tregs. Additionally, it has been suggested that Tregs play a role in maintaining epithelial barrier via the production of TGF‐β and IL‐10. Finally, probiotics are able to modulate the Th1 and Th2 responses, resulting in the restoration of the immune homeostasis. DCs, dendritic cells; Tregs, regulatory T cells; PRRs, pattern recognition receptors; TLRs, Toll‐like receptors; CLRs, C‐type lectin receptors; NLRs, NOD‐like receptors; LTA, lipoteichoic acid; CPS, cell wall–associated polysaccharide; LPS, lipopolysaccharide
Interestingly, lactobacilli were shown to be normal inhabitants of the healthy nasopharynx and upper respiratory tract (URT).27-29 For instance, Bogaert et al27 showed a predominance of Lactobacillus species in children in summer (absolute abundance is 96%) compared to winter (absolute abundance is 10%). Additionally, lactobacilli were also detected in the healthy nasopharynx of Chinese individuals30 and in the nasopharynx of healthy Belgian individuals (up to 10% relative abundance).31 Lactobacilli are also found in the tonsillar crypts of both healthy adults and infants.32 Nevertheless, whether lactobacilli could also function as probiotics in the nasopharyngeal niche is currently underexplored.
In this review, we summarize documented beneficial effects of probiotic microorganisms (mainly Lactobacillus and other species) and discuss their potential mechanisms of action in chronic diseases of the upper and lower airways. As the studies of the gastrointestinal impact of probiotics are more advanced, some data from this field are incorporated as additional supporting evidence. We describe documented barrier‐restoring capacities of probiotics, their mechanisms of interaction with the innate immune system, and the potential clinical applications of these bacteria to treat different chronic inflammatory diseases of the upper URT.
2 PROBIOTIC REGULATION OF THE EPITHELIAL BARRIER FUNCTION
The epithelial barrier is the first physical barrier protecting the human body from the entrance of harmful substances including allergens, pathogens, and irritants.33 Intercellular junctions, such as tight junctions (TJs), adherence junctions (AJs), and desmosomes, play a critical role in the formation and maintenance of the physical barrier. TJs are the most apically located junctions and provide the epithelia with a semipermeable size‐ and ion‐specific barrier, restricting diffusion of macromolecular components.18, 34, 35 AJs are positioned immediately below TJs and are essential for cell‐cell adhesion, regulation of the actin cytoskeleton, intracellular signaling, and transcriptional regulation in epithelial cells.36 Desmosomes form the third intercellular junction complex and are important in maintaining barrier integrity and cell adhesion.37
The beneficial effects of probiotics on epithelial barrier dysfunction have been extensively studied in the gastrointestinal tract (Table 1). For instance, Lactobacillus plantarum MB452 has been shown to increase the expression levels of TJ‐related genes in healthy intestinal epithelial cells (IECs) in vitro.38 Similar positive effects on intestinal epithelial barrier integrity and TJ expression were observed with other probiotic strains such as Lactobacillus rhamnosus GG,39 Streptococcus thermophiles ATCC19258, L. plantarum MB452,40 and the Gram‐negative probiotic strain Escherichia coli Nissle 1917.41, 42 Additionally, certain Lactobacillus strains appear to have the capability to increase epithelial barrier integrity through the stabilization of AJs expression.43 More specifically, the lactobacilli tested could increase the expression of E‐cadherin and β‐catenin and decrease the abundance of protein kinase C (enzyme involved in the disassembling of AJs) in T84 human colonic epithelial cell line.43 Various barrier‐restoring properties of probiotics have also been demonstrated in different in vivo models. For instance, in a murine model of colitis induced by dinitrobenzene sulfonic acid, oral administration of L. rhamnosus CNCM I‐3690 protected epithelial barrier integrity by modulating TJ (occludin) and AJ molecules (E‐cadherin).44
Table 1. Barrier‐restoring capacities of different probiotics in the gastrointestinal tract
Strain TJ or AJ proteins Cells or mouse model Barrier function Ref.
L. plantarum MB452 ↑ OCCL, ZO‐1, ZO‐2, and cingulin expression Caco‐2 cells Increase in barrier integrity 38
L. rhamnosus GG ↑ ZO‐1, claudin‐1, and OCCL expression Caco‐2 cells Restoration of gliadin induced epithelial barrier disruption 39
S. thermophiles ATCC19258 and L. acidophilus ATCC4356 Maintenance of actin and ZO‐1, ↑ actinin and OCCL expression HT29/cl.19A and Caco‐2 cells Increase in barrier function of untreated epithelial cells 40
E. coli Nissle 1917 ↑ ZO‐1 expression Human small and large intestinal epithelial cells and DSS‐treated mice Upregulation of TJ expression in murine IECs and protection against DSS colitis induced increase in mucosal permeability 41
E. coli Nissle 1917 ↑ ZO‐2 expression, silencing of PKCδ T84 cells Restoration of a disrupted epithelial barrier 42
L. acidophilus, L. fermentum, L. gasseri and L. rhamnosus ↑ Stabilization of E‐cadherin and β‐catenin T84 cell monolayers Increase in barrier integrity 43
L. rhamnosus CNCM I‐3690 ↑ OCCLN and E‐cadherin expression Caco‐2 cells and murine model of colitis induced by DNBS Restoration of intestinal barrier function and increased levels of TJ and AJ proteins 44
In the airways, there are currently limited reports describing the regulatory properties of probiotics on the epithelial barrier. In vivo, it has been shown that oral treatment with L. rhamnosus CRL1505 could prevent the polycytidylic acid [poly (I:C)]‐induced increased permeability of the bronchoalveolar‐capillarity barrier, as determined by albumin levels in the lungs.45 This improvement was linked to a decrease in the activation of pro‐inflammatory cells and the production of pro‐inflammatory cytokines in the lungs.45 Similar findings were observed using nasally administered Lactococcus lactis NZ9000, which could counteract S. pneumoniae‐induced lung tissue permeability.46 In vitro, the stimulation of Calu‐3 bronchial epithelial cells with the synthetic bacterial lipopeptide Pam3CysSK4 resulted in a concentration‐dependent increase in epithelial barrier function and decrease in epithelial permeability, as a result of an increased expression of the TJ proteins claudin‐1 and Zonula occludens (ZO) ‐1 and a decreased expression of occludin.47
3 PROBIOTIC INTERACTIONS WITH THE IMMUNE SYSTEM
3.1 Interaction with pattern recognition receptors
Probiotics can modulate local and systemic host immune responses in a strain‐specific manner via the expression of different microorganism‐associated molecular patterns (MAMPs), such as lipopolysaccharides (LPS), flagellin, CpG‐DNA, and other surface (lipo)proteins.5 MAMPs are recognized by pattern recognition receptors (PRRs) present on epithelial and innate immune cells (Figure 2, Table 2). The best‐studied PRRs are the Toll‐like receptors (TLRs), NOD‐like receptors (NLRs), and C‐lectin type receptors.48 Thanks to the broad specificity of PRRs, a limited number of them can detect a wide range of MAMPs.48 Interaction between MAMPs and PRRs leads to the activation of multiple signaling cascades that generates an appropriate molecular response against the encountered microorganism.49, 50 In a 56‐day murine ovalbumin (OVA)‐induced asthma model, it has been demonstrated that oral treatment with L. rhamnosus NutRes1 and Bifidobacterium breve M‐46 was able to modulate the mRNA expression levels of Tlr9 and Tlr3.51 However, these results need to be analyzed with prudence, because the increase in mRNA expression levels of Tlr3 and Tlr9 does not necessarily prove that these 2 probiotics were recognized directly by the receptors. A similar reduction in the type 1 immune response and injury in the lungs were observed in a murine model of sepsis where treatment with L. rhamnosus GG and Bifidobacterium longum (strain not specified) via oral gavaging could attenuate neutrophil infiltration in the lungs and decreased the expression of interleukin (IL)‐6 and tumor necrosis factor‐α (TNF‐α). The beneficial effects of these probiotics appeared to be associated with decreased gene expression levels of TLR2 and MyD88 in the lungs of the mice.52 Finally, oral treatment with Lactobacillus reuteri ATCC 23272 of OVA‐sensitized mice has been shown to result in attenuation of inflammatory cell influx to the lungs and airway hyper‐reactivity (AHR). These beneficial effects seemed to be dependent on TLR9, because the treatment of TLR9−/− mice with this L. reuteri strain did not attenuate inflammatory cell influx in the lungs or AHR. Additionally, the attenuation of inflammatory cell influx was associated with reduced levels of pro‐inflammatory cytokines such as IL‐5 and IL‐13 in the bronchoalveolar lavage fluid (BALF) of the mice. However, L. reuteri ATCC 23272 could not alter Th1‐type cytokines IL‐12 and IFN‐γ or the anti‐inflammatory cytokine IL‐10 in BALF.53 Moreover, the authors showed that these effects were strain‐specific, because oral treatment with Lactobacillus salivarius AH102 did not improve allergic airway response in the OVA‐sensitized mice.53 This is in agreement with the fact that the sum of all MAMPs expressed by a certain probiotic is strain‐specific, resulting in their differential effects on epithelial and/or immune cells.5, 54
Table 2. Examples of interactions of probiotics with the immune system (relevant for respiratory allergies)
Strain Mechanism Outcome Ref.
L. rhamnosus NutRes1 and Bifidobacterium breve M‐46 Reduction of chronic allergic inflammation by increasing the mRNA expression levels of Tlr9 and Tlr3 Suppression of pulmonary airway inflammation, induction of airway remodeling and inhibition of mast cell degranulation (48)
L. rhamnosus GG and Bifidobacterium longum ATCC Attenuation of neutrophil infiltration in the lungs and decreased expression of IL‐6 and TNF‐α via decreased gene expression of TLR2 and MyD88 Reduction of immune response and lung injury (49)
L. reuteri ATCC 23272 Modulation of TLR9 resulted in reduced levels of pro‐inflammatory cytokines such as IL‐5 and IL‐13 Attenuation of inflammatory cell influx to the lungs and AHR (50)
L. reuteri and L. casei Modulation of DC function through dendritic cell‐specific intercellular adhesion molecule 3‐grabbing non‐integrin in vitro Induction of IL‐10 producing Tregs in vitro (67)
L. rhamnosus GG and B. lactis Bb‐12 Induction of Tregs, associated with increased expression of TGF‐β in vivo Inhibition of allergic sensitization and airway disease in an asthma mouse model (64)
L. casei IBSO41 and L. acidophilus D031 Stimulation of DCs in vitro Increased production of TGF‐β (69)
L. plantarum NCIMB8826 Inhibition of specific IgE response, high levels of specific IgG2a and increased production of IFN‐γ Inhibition of an allergic Th2 profile (63)
L. rhamnosus GG Significant decrease in eosinophils, IL‐13 and IL‐5 levels in the lungs, and airway hyperactivity Prevention of birch‐pollen‐induced allergic asthma by LGG (62)
To our knowledge, there is currently no in vitro evidence showing the interaction between probiotics and PRRs using primary nasal or bronchoepithelial cells. Nonetheless, the modulating effects of TLRs by probiotics have been investigated using intestinal cell lines. For example, Ganguli et al55 have shown the anti‐inflammatory effects of L. rhamnosus GG and its SpaC pilus adhesin through the modulation of the TLR/IL‐1R signaling pathway. The investigators demonstrated that T84 cells stimulated with Salmonella enterica serovar Typhimurium SL1344 or LPS can reduce IL‐1β and IL‐6 secretion when treated with L. rhamnosus GG. These effects were due to the binding of SpaC pilus adhesin to the IECs, resulting in the downregulation of the mRNA levels of TLR3 and TLR4.55 In addition, phenotypic comparison of wild‐type and pili mutant strain of L. rhamnosus GG showed that the presence of pili reduced IL‐8 induction in Caco‐2 cells, which was at least partially mediated by TLR2.56 As the innate immune cells expressing PRRs provide a link between the innate and adaptive immunity, the described effects can further translate into the modulation of different T‐cell subsets and the corresponding cytokines.
3.2 Modulation of Th1/Th2 and regulatory T‐cell immune response by probiotics
Probiotic applications have been shown to impact effector and regulatory T‐cell responses in airway disease, resulting in the restoration of the Th1/Th2 immune balance,57 and/or stimulation of regulatory cytokine production and the regulatory T‐cell (Treg) subset.58-61 This typically leads to a reduction in lung Th2 inflammatory cytokine levels (eg, IL‐5 and IL‐13) and the attenuation of AHR, as recently demonstrated for preventive intranasal administration of L. rhamnosus GG in a mouse model of birch pollen–induced allergic asthma.62 Additional immunomodulatory properties of probiotics in airway disease, including the stimulation of mucosal IgA levels and allergen‐specific B‐ and T‐cell responses, are well described elsewhere.63
Allergic airway disease is typically a result of excessive Th2 reactions; therefore, the ability of probiotic strains to stimulate a counterbalancing Th1 immune response can be a valuable mechanism of airway disease prevention and treatment. In vitro, co‐incubation with L. plantarum NCIMB885 induced a Th1‐biased cytokine profile in murine dendritic cells (DCs), which could be linked to lipoteichoic acid composition of this probiotic strain.64 In vivo, orally administered L. rhamnosus GG led to an increase in Th1 (IL‐12 and IFN‐γ) and a decrease in Th2 (IL‐4, IL‐5, and IL‐13) BALF cytokine levels in a mouse model of OVA‐induced airway inflammation.65 This led to a reduction in inflammatory cell counts in the BALF (ie, eosinophils, lymphocytes, and monocytes) and AHR.65 The development of a Th1 over Th2 response was likewise observed as a result of intranasal co‐application of L. plantarum NCIMB8826 and the clinically relevant house dust mite aeroallergen Der p 1 in a mouse model of Der p 1 sensitization.66 This was reflected in an increase in specific IgG2a levels, a decrease in IFN‐γ production by restimulated splenocytes, and an inhibition of specific IgE production. These effects were accompanied by the attenuation of airway inflammation after house dust mite extract challenge, as demonstrated by reduced BALF eosinophil counts.66
Marked improvement of airway disease symptoms in murine models as a result of probiotic administration has also been linked to the induction of Tregs.67, 68 Tregs are a subset of T lymphocytes, pivotal in maintaining immune homeostasis through the suppression of self‐reactive T‐cell activation and expression.69 Their function is tightly linked to the regulatory IL‐10 and transforming growth factor (TGF)‐β cytokines, which can drive the activation and proliferation of Tregs along with their suppressive functions. These regulatory cytokines can be produced by DCs58-60 or T cells61 in response to interactions with probiotic strains. For example, L. reuteri ASM20016 and Lactobacillus casei NIZO B255 have been shown to bind DC‐SIGN on DCs, which in turn drove the development of IL‐10‐producing Tregs.70 Also, for L. rhamnosus GG, it was recently shown that glycosylated heteropolymeric pili are involved in such DC‐SIGN‐mediated interactions with DCs.71 In another study, Bifidobacterium lactis AD011 increased the production of IL‐10 in a co‐culture with DCs and IECs, whereas L. casei IBS041 and Lactobacillus acidophilus AD031 increased TGF‐β secretion in this co‐culture system.72 Similar results were obtained in another co‐culture system containing peripheral blood mononuclear cells and L. lactis, that is, an increased secretion of the anti‐inflammatory cytokine IL‐10.73 The in vitro data are further supported by in vivo studies in mouse models of allergic airway disease. Feleszko et al67 have shown that orally administered L. rhamnosus GG or B. lactis Bb‐12 can inhibit subsequent allergic sensitization and airway disease symptoms in a murine model of OVA‐induced asthma. These effects are partially due to the induction of Foxp3‐expressing Treg cells in the peribronchial lymph nodes, as well as increased TGF‐β production by T cells in the mesenteric lymph nodes67. Likewise, oral treatment with L. rhamnosus NutRes1 and B. breve M‐46 stimulated Foxp3 expression in blood Treg cells and decreased the levels of various pro‐inflammatory cytokines such as IL‐6 and IL‐4 in the thoracic lymph nodes in a murine OVA‐induced asthma model.51 This resulted in beneficial effects to the same extent as the corticosteroid budesonide, that is, suppression of pulmonary airway inflammation, the induction of airway remodeling, and the inhibition of mast cell degranulation.74 Also, intranasal application of probiotic strains, such as L. paracasei NCC2461, has been shown to increase lung CD4+CD25+FoxP3+ Treg cell counts in a mouse model of allergic airway inflammation and to attenuate the allergic response.75
Taken together, these studies emphasize the ability of certain probiotic strains to shift the immune balance away from pathological responses, suggesting their potential application for prevention and/or treatment of airway inflammatory diseases.
4 CLINICAL APPLICATION OF PROBIOTICS FOR THE AIRWAYS
The beneficial effects of probiotics are highly influenced by different parameters such as duration and route of administration as well as the type of strain. Various studies have used murine models of allergic airway diseases to investigate the effects of probiotics. The administration route of these probiotics is typically oral, via drinking water or food,76-78 or through intragastric intubation.75 More recently, intranasal administration of probiotic bacteria has been increasingly explored in different mice studies.62, 75, 79 In the study of Spacova et al, the authors demonstrated that intranasal administration of L. rhamnosus GG attenuated birch pollen–induced asthma in mice.62 However, when these mice were intragastric pretreated with L. rhamnosus GG, there was no significant effect on allergic sensitization.62 The effectiveness of intranasal application was also demonstrated in the study of Pellaton et al75 Their findings demonstrated that intranasal application of L. paracasei NCC2461 can modify both the systemic and local immune response and allows for niche‐specific effects compared to oral treatment. Also, clinical trials in humans to investigate the effects of probiotics on the airways are recently booming.80 In most clinical trials, the probiotics are orally administered, for instance via capsules or supplemented in dairy products, and exert their effects mostly via modulation of the immune system. However, based on the results obtained in different murine models, we believe that local administration (eg, nasal spray) can induce more niche‐specific effects both in the upper and in the lower airways, leading to a more effective treatment.
4.1 Probiotics for the upper respiratory tract diseases
Different randomized placebo‐controlled trials have been performed to investigate the potential of oral administration of probiotics in upper airway diseases.80, 81 In the meta‐analysis of Peng et al82, authors analyzed 11 randomized controlled trials in which probiotic efficiency for the prevention and/or treatment of allergic rhinitis (AR) was investigated. Results showed that in these 11 trials, probiotic treatment resulted in the improvement of the quality of life and nasal symptoms of the patients. However, none of the probiotic strategies tested could prevent AR nor improve the inflammatory markers in these patients. In the more recent meta‐analysis of Guvenc et al,81 22 randomized double‐blind placebo‐controlled studies were included, of which 17 studies demonstrated an improvement of the clinical symptoms in AR patients such as nasal blockage, rhinorrhea, and nasal itching. These beneficial effects were in 8 of the included trials linked with a decrease in inflammatory markers.81 The combination of different strains has also been studied in patients with AR. For instance, Koyama and colleagues tested a probiotic yoghurt containing a mixture of L. rhamnosus GR1 and Bifidobacterium adolescentis in 36 subjects with AR. They found no significant effects on clinical outcomes of the participants, but did observe increased serum levels of IL‐10 and IL‐12 during grass pollen season, and increased TGF‐β in ragweed season in the probiotic group compared to the placebo group.83 Thus, although no effects on the patients' symptoms were observed, important regulatory markers were increased in these patients after probiotic administration.
Although the above‐mentioned studies indicate that probiotics may improve the overall quality of life and nasal symptoms of AR patients, strong evidence is still lacking to substantiate preventive and/or therapeutic roles for probiotics in AR. Moreover, nasal application of probiotics has not yet—to the best of our knowledge—been tested in AR patients. Nasal application of probiotics can lead to the modulation of inflammation in the lower airways which was shown in a mouse model of allergic airway inflammation.75 These results are very promising because asthma is an important comorbidity of AR.84
In contrast to AR, the use of probiotics in patients with CRS is even less explored. In a small‐scale pilot study, patients with CRS were shown to have reduced bacterial diversity with specific depletion of beneficial lactobacilli in brush samples of mucosal surfaces of the lateral, central, and medial portions of the maxillary sinuses.85 Interestingly, these same authors could subsequently show that Lactobacillus sakei ATCC 15521 was sufficient to protect against Corynebacterium tuberculostearicum‐induced sinusitis after intranasal inoculation in mice.85 The authors focused on L. sakei because they found this species in higher abundances in the mucosa of healthy subjects and it was among the taxa that were significantly depleted in CRS patients.85 This was one of the first studies to demonstrate that specific enrichment of the URT with lactobacilli can result in significant health benefits. In humans, a randomized placebo‐controlled trial in CRS patients that were treated orally for 4 weeks with L. rhamnosus R0011 showed no improvement in sinonasal quality of life scores (SNOT‐20 questionnaire).86 Another double‐blind placebo‐controlled multicenter study demonstrated that oral application of Enterococcus faecalis Symbioflor 1 for 6 months could result in a reduction in the frequency of acute exacerbations of CRS. Furthermore, these beneficial effects were sustained for 8 months after the end of the treatment.87 In line with these results, Kitz et al have reported a reduction in frequency and duration of recurrent acute sinusitis in children treated orally with E. faecalis Symbioflor‐1 for 8 weeks.88
To the best of our knowledge, only 1 placebo‐controlled trial has investigated the potential of a nasal spray for the treatment of CRS without nasal polyps.79 In this trial, 20 patients received a nasal spray containing 13 honeybee lactic acid bacteria (LAB) species (nine Lactobacillus and 4 Bifidobacterium species) for 2 weeks. After 2 weeks, the symptoms of the patients (SNOT‐22 questionnaire) were evaluated, together with inflammatory markers in nasal lavage fluid and their microbiome. This study proved that the nasal LAB spray was well tolerated by the patients without any side effects.79 However, there was no significant improvement with these honeybee isolates in symptom severity, nor changes in the immune response or microbiome of the patients.79 Nevertheless, this study is a first important step toward the use of nasal probiotic sprays for the treatment of CRS. Also, other studies exploring the use of a nasal spray containing probiotics for other URT infections, such as otitis media, already reported the safety of a probiotic spray in the nasal cavity.24, 89, 90 Additionally, we believe that better strain selection (especially human isolates from the URT) might be needed to observe significant effects on the clinical symptoms of patients.
4.2 Probiotics for the lower respiratory tract diseases
When studying the treatment potential of probiotics in chronic inflammatory diseases of the URT (eg, AR and CRS), it is important to consider that these diseases are often associated with comorbidities in the lower airways, that is, mainly asthma.84 Clinical studies investigating the potential of probiotics to treat patients with asthma are preliminary but show some promising results. For instance, Chen et al performed a randomized, double‐blind, placebo‐controlled trail to investigate the clinical potential of Lactobacillus gasseri PM‐A0005 in children (6‐12 years old) with asthma.91 After 2 months of daily treatment with 2x109 CFU/capsule of L. gasseri PM‐A0005, they measured an improvement in asthma symptom score, together with an improvement in airway functions, that is, peak expiratory flow rates.91 Additionally, they observed a significant reduction in the blood levels of pro‐inflammatory cytokines in the patients, such as TNF‐a, IFN‐γ, IL‐12, and IL‐13.91 In line with these results, Gutkowski et al showed that oral treatment with Trilac, a mixture containing Lactobacillus acidophilus, Lactobacillus delbruecki ssp. bulgaricus, and Bifidobacterium bifidum, for 12 weeks in children with mild‐to‐moderate atopic asthma, resulted in an improvement in lung function and a reduction in exacerbations of asthma.92 In another study, van de Pol et al investigated the effect of synbiotic treatment in patients with asthma and house dust mite allergy.93 They demonstrated that treatment with the combination of B. breve M‐16V and galacto‐ and fructo‐oligosaccharide for 4 weeks had no effect on allergen‐induced changes in sputum eosinophils or lung function in these patients. However, a significant improvement in the baseline lung function and IL‐5 levels in the serum was observed.93 Finally, in the study of Rose et al, the authors performed a placebo‐controlled clinical study where they investigated 6 months of L. rhamnosus GG food supplementation in infants with atopic predisposition and recurrent wheezing.94 The results demonstrated no improvement in clinical outcomes, measured via severity scoring of atopic dermatitis and asthma symptom score (Ref Rose et al 2010). However, oral treatment with L. rhamnosus GG resulted in mild reduction in sensitization to aeroallergens as predictor for bronchial asthma.94 More information regarding the involvement of airway microbial dysbiosis in asthma and the potential of probiotics to treat these patients are extensively reviewed elsewhere.95
Although these studies are promising, more research is needed to evaluate whether targeting the URT via nasal and/or oral probiotic treatment is efficient enough to modulate the lung microbiota.
5 CONCLUDING REMARKS
As the first culture‐independent report on the presence of a healthy lung microbiota,96 studies demonstrated the complex microbial communities residing in both the upper and the lower airways of healthy individuals.97, 98 A dysbiosis of the microbiota has been shown in chronic airway diseases which, interestingly, seems to occur concomitantly with a defective epithelial barrier, as observed in asthma,19 AR,18, 99 and CRS.17 Therefore, we believe that probiotics will represent a novel treatment option for chronic airway diseases. Probiotics can modulate nasal epithelial barrier via modulation of TJs and AJs and interact with the innate immune system, resulting in the restoration of mucosal barrier integrity.
Furthermore, the nature and extent of these beneficial effects might vary depending on the probiotic strain and administration route. Recent studies point toward the niche‐specific effect of nasal administration of probiotics. Further research is nevertheless warranted to investigate the potential of nasal administration for the treatment of upper and lower airway diseases.
In addition, most of the results are obtained using in vitro and in vivo models of the intestine, and therefore, further research is needed to prove the beneficial effects of probiotics in the upper and lower airways.
Probiotics for the airways: Potential to improve epithelial and immune homeostasis - Martens - 2018 - Allergy - Wiley Online Library
https://onlinelibrary.wiley.com/doi/full/10.1111/all.13495
The American Journal of Pathology
Volume 187, Issue 3, March 2017, Pages 476-486
The American Journal of Pathology
Changes in the Luminal Environment of the Colonic Epithelial Cells and Physiopathological Consequences
Author links open overlay panelFrançoisBlachier∗MartinBeaumont∗MireilleAndriamihaja∗Anne-MarieDavila∗AnnaïgLan∗MartaGrauso∗LucieArmand∗RobertBenamouzig∗†DanielTomé∗
UMR Physiologie de la Nutrition et du Comportement Alimentaire, AgroParisTech, INRA, Université Paris-Saclay, Paris, France
†
Department of Gastroenterology, Avicenne Hospital, Assistance Publique-Hôpitaux de Paris, Bobigny, France
Evidence, mostly from experimental models, has accumulated, indicating that modifications of bacterial metabolite concentrations in the large intestine luminal content, notably after changes in the dietary composition, may have important beneficial or deleterious consequences for the colonic epithelial cell metabolism and physiology in terms of mitochondrial energy metabolism, reactive oxygen species production, gene expression, DNA integrity, proliferation, and viability.Recent data suggest that for some bacterial metabolites, like hydrogen sulfide and butyrate, the extent of their oxidation in colonocytes affects their capacity to modulate gene expression in these cells. Modifications of the luminal bacterial metabolite concentrations may, in addition, affect the colonic pH and osmolarity, which are known to affect colonocyte biology per se. Although the colonic epithelium appears able to face, up to some extent, changes in its luminal environment, notably by developing a metabolic adaptive response, some of these modifications may likely affect the homeostatic process of colonic epithelium renewal and the epithelial barrier function. The contribution of major changes in the colonocyte luminal environment in pathological processes, like mucosal inflammation, preneoplasia, and neoplasia, although suggested by several studies, remains to be precisely evaluated, particularly in a long-term perspective.
Changes in the Luminal Environment of the Colonic Epithelial Cells and Physiopathological Consequences - ScienceDirect
https://www.sciencedirect.com/science/article/pii/S0002944016305429
Published: 14 July 2017
(Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease
Thomas W. Buford
Microbiome volume 5, Article number: 80 (2017) Cite this articleDepartment of Medicine, University of Alabama at Birmingham
Abstract
Chronic inflammation represents one of the most consistent biologic features of aging. However, the precise etiology of persistent low-grade increases in inflammation remains unclear. Recent evidence suggests that the gut microbiome may play a key role in age-related inflammation. Indeed, several studies have indicated that older adults display an altered composition of the gut microbiota, and early evidence indicates that this dysbiosis is associated with the presence of several key circulating inflammatory analytes. The present review summarizes knowledge on age-related inflammation and discusses how potential relationships with gut dysbiosis may lead to novel treatment strategies in the future.
“The pattern of disease is an expression of the response of man to his total environment (physical, biological, and social); this response is, therefore, determined by anything that affects man himself or his environment.” – Rene Dubos, 1961(Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease | Microbiome | Full Text
https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-017-0296-0
Alzheimers Res Ther. 2018; 10: 124.
The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease
Background
Trimethylamine N-oxide (TMAO), a small molecule produced by the metaorganismal metabolism of dietary choline, has been implicated in human disease pathogenesis, including known risk factors for Alzheimer’s disease (AD), such as metabolic, cardiovascular, and cerebrovascular disease.
Methods
In this study, we tested whether TMAO is linked to AD by examining TMAO levels in cerebrospinal fluid (CSF) collected from a large sample (n = 410) of individuals with Alzheimer’s clinical syndrome (n = 40), individuals with mild cognitive impairment (MCI) (n = 35), and cognitively-unimpaired individuals (n = 335). Linear regression analyses were used to determine differences in CSF TMAO between groups (controlling for age, sex, and APOE ε4 genotype), as well as to determine relationships between CSF TMAO and CSF biomarkers of AD (phosphorylated tau and beta-amyloid) and neuronal degeneration (total tau, neurogranin, and neurofilament light chain protein).
Results
CSF TMAO is higher in individuals with MCI and AD dementia compared to cognitively-unimpaired individuals, and elevated CSF TMAO is associated with biomarkers of AD pathology (phosphorylated tau and phosphorylated tau/Aβ42) and neuronal degeneration (total tau and neurofilament light chain protein).
Conclusions
These findings provide additional insight into gut microbial involvement in AD and add to the growing understanding of the gut–brain axis.The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6303862/
The Gut, the Heart, and TMAO
CLEVELAND HEARTLAB AUGUST 1, 2016 HEART ATTACK AND STROKE, TMAO
Dietary Choline-Gut Microbiome,The Surprising Link Between the Gut and Heart Health
Science has long recognized that what we eat plays a critical role in our heart health. Now the details of this complex connection are coming into focus.
One of the more intriguing recent discoveries has to do with the role of the gut microbiome—the trillions of microbes that reside in the GI tract and influence health by helping digest food, making vitamins, and providing protection against disease-causing microorganisms. Recently Cleveland Clinic researchers reported findings from several studies involving people and animals, that the gut microbiome directly changes the function of blood platelets, influencing the risk for heart attack and stroke.
Here’s how it works: When people ingest certain nutrients, such as choline (abundant in red meat, egg yolks, and dairy products) and L-carnitine (found in red meat as well as some energy drinks and supplements), the gut bacteria that break it down produce a compound called trimethylamine (TMA). The liver then converts TMA into the compound, trimethylene N-oxide (TMAO).
The trouble with TMAO is that data show high levels contribute to a heightened risk for clot-related events such as heart attack and stroke—even after researchers take into account the presence of conventional risk factors and markers of inflammation that might skew the results. In their most recent analysis, scientists showed that high blood levels of TMAO were associated with higher rates of premature death in a group of 2235 patients with stable coronary artery disease. Those found to have higher blood levels of TMAO had a four-fold greater risk of dying from any cause over the subsequent five years.
The implications are intriguing. Taken together, the new studies suggest that positively altering the gut microbiota may help to reduce damage to blood vessels, resulting in a stronger cardiovascular system, and they point to targets for potential new heart disease therapies.
The insights also underscore the potential power of TMAO testing, which can help patients determine if their gut microbiome is contributing to their risk for heart disease and whether they might benefit from limiting foods that contain the building blocks of TMAO. TMAO tests are only available through the Cleveland Heartlab.
To lower your TMAO levels, consider minimizing the consumption of full-fat dairy products, including whole milk, egg yolk, cream cheese, and butter; both processed and unprocessed red meat (beef, pork, lamb, and veal), as well as nutritional supplements and energy drinks containing choline, phosphatidylcholine (lecithin), and/or L-carnitine. Vegetarians and vegans, who avoid meat products, for instance, produce little TMAO.
In general, consuming a diverse diet rich in plant foods and fiber may be helpful. When Cleveland Clinic researchers fed mice a diet rich in TMAO producing nutrients, they identified a compound called DMB capable of minimizing TMAO produced from their gut microbiota. In fact, when DMB was added to their drinking water, they found TMAO levels and the formation of arterial plaques both declined. DMB may be found naturally in many Mediterranean diet foods, including red wine and extra virgin olive oil.
Such an eating pattern may turn out to be key to cultivating a healthy human gut microbiome—one that will fend off myriad illnesses, including heart disease, America’s chief health threat.The Gut, the Heart, and TMAO - Cleveland HeartLab, Inc.
https://www.clevelandheartlab.com/blog/the-gut-the-heart-and-tmao/
Could Food Be a New Medicine to Fight Heart Disease?
CLEVELAND HEARTLAB JANUARY 11, 2016 DIET, HEART ATTACK AND STROKE, METABOLIC SYNDROME
A compound called DMB (3,3-dimethyl-1-butanol), found in olive oil, red wine and other foods, may someday be a first-of-its kind drug with the potential to treat—or even prevent—heart disease in the future, suggests a new Cleveland Clinic study published in the journal Cell.
The investigators report that in mice, dietary supplementation with this naturally occurring compound safely inhibited atherosclerosis (plaque buildup in arteries) and significantly lowered production of TMAO (trimethylamine-N-oxide), a heart-harming metabolite derived from gut bacteria.
If future studies show similar effects in people, the research may ultimately lead to a new strategy to treat—or even prevent—heart disease and stroke, two of the leading causes of death globally. To date, no trials of DMB have been done in humans.
“This study shows for the first time that one can target a gut microbial pathway to inhibit atherosclerosis,” says senior study author Stanley Hazen, MD of the Cleveland Clinic. “This new approach opens the door to the concept of drugging the microbiome to affect a therapeutic benefit in the host.”Could Food Be a New Medicine to Fight Heart Disease? - Cleveland HeartLab, Inc.
https://www.clevelandheartlab.com/blog/horizons-could-food-be-a-new-medicine-to-fight-heart-disease/
3,3-Dimethyl-1-butanol (DMB) is a structural analog of choline.[1]
Effects
DMB inhibits microbial trimethylamine (TMA) formation in mice and in human feces, thereby reducing plasma trimethylamine N-oxide (TMAO) levels after choline or carnitine supplementation.[1] It consequently inhibited choline-enhanced endogenous macrophage foam cell formation and atherosclerotic lesion development in mice without alterations in circulating cholesterol levels.[1]
While mice placed on a choline supplemented diet showed an increase in the proportions of the bacterial taxon Clostridiales in the gut, DMB induced a decrease in the proportions of this taxon.[1]
Mice showed no evidence of toxicity to chronic (16-week) DMB exposure.[1]
3,3-二甲基-1-丁醇(DMB)是胆碱的结构类似物。[1] 特效 DMB抑制小鼠和人类粪便中微生物三甲胺(TMA)的形成,从而降低补充胆碱或肉碱后血浆三甲胺N-氧化物(TMAO)的水平。[1]因此,它抑制了小鼠胆碱增强的内源性巨噬细胞泡沫细胞的形成和动脉粥样硬化病变的发展,而胆固醇水平却没有改变。[1] 接受胆碱补充饮食的小鼠肠道内细菌分类群梭状芽胞杆菌的比例增加,而DMB诱导该分类群比例降低。[1] 小鼠未显示对慢性(16周)DMB暴露有毒性的证据。[1]3,3-Dimethyl-1-butanol (choline analogue) as a TMAO inhibitor - Supplements - LONGECITY
https://www.longecity.org/forum/topic/103257-33-dimethyl-1-butanol-choline-analogue-as-a-tmao-inhibitor/
EPA and DHA for IBD
4. Inflammatory Bowel Disease (IBD)
The same or similar story is being described in the research literature last year or this year for essentially all of the inflammation-related diseases. The 2016 publication Inflammatory bowel disease: can omega-3 fatty acids really help? relates: “Adjuvants to the traditional therapy of inflammatory bowel disease (IBD) have been studied to enhance the efficacy of the treatment and improve patients’ quality of life. Omega-3 polyunsaturated fatty acids (ω3FA) have been associated with attenuation of the inflammatory responses in IBD, possibly acting as substrates for anti-inflammatory eicosanoid production, similar to prostaglandins and leukotrienes. ω3FA also act as substrates for the synthesis of resolvins, maresins and protectins, indispensable in resolving inflammation processes. These acids may influence the development or course of IBD by: reducing oxidative stress, production of tumor necrosis factor-α and proinflammatory cytokines; working as chemopreventive agents; and decreasing the expression of adhesion molecules. There are numerous controversies in the literature on the effects of ω3FA in the prevention or treatment of IBD, but their effects in reducing inflammation is incontestable. Therefore, more studies are warranted to elucidate the pathophysiological mechanisms and establish the recommended daily intake to prevent or induce remission in IBD patients.”
This 2016 publication relates to inflammatory bowel disease, EPA- and DHA-derived resolvins’ actions in inflammatory bowel disease. “Inflammatory bowel diseases are chronic diseases divided into two major forms, ulcerative colitis and Crohn’s disease, which are both associated with a chronic inflammatory condition of the gastrointestinal tract. Recent studies have shown that the resolution of inflammatory conditions is a biosynthetically active process where new pro-resolution lipid mediators derived from omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), such as E- and D-series resolvins, protectins, and macrophage mediator in resolving inflammation (maresins), have potent anti-inflammatory activity and serve as specialised mediators that play an important role in the resolution of inflammation. Recent studies have also shown the role of resolvins in referred hyperalgesia associated with different inflammatory processes, such as the visceral pain caused by inflammatory bowel disease. There are many reports describing the principal effects of EPA- and DHA-derived mediators in experimental models of inflammatory bowel diseases. This review focuses on the recent studies on the important role played by pro-resolution lipid mediators in controlling the inflammatory process associated with inflammatory bowel diseases.”
Types of Diarrhea
There are 3 basic categories of diarrhea: watery, fatty (malabsorptive) and inflammatory. Not all diarrhea is purely watery, malabsorptive, or inflammatory because some categories overlap. Also, individuals may experience diarrhea which has multiple causes, thus presenting a more complex situation.
1. Watery Diarrhea
Watery diarrhea is characterized by runny or watery bowel movements. There are 3 subcategories of watery diarrhea: osmotic, secretory and functional.
Osmotic Diarrhea
Osmotic diarrhea occurs when something is drawing water from the rest of the body into the bowels. In most cases, osmotic diarrhea will cease when one stops ingesting the food or substance that is causing water to be drawn into the bowels.
Secretory Diarrhea
Secretory diarrhea involves a higher stool volume, diarrhea that often occurs through the night and will continue even if one in fasting.
Secretory diarrhea occurs when more water is released into the bowels than is usual. This happens due to an increase in secretion or a decrease in absorption of electrolytes in the bowels. There are a variety of causes for this disruption.
Functional Diarrhea
Functional diarrhea involves hypermotility (excessive movement of the muscles in the GI tract) and is characterized by smaller volumes of diarrhea. Functional diarrhea improves at night and with fasting. Functional diarrhea does not have any associated abdominal pain.
People with functional diarrhea don’t usually have it all the time. Normal bowel movements, and even constipation can occur between bouts of diarrhea.
Functional Diarrhea has no know cause. There are no structural or biochemical abnormalities with this type of diarrhea, part of the GI system just does not function quite right.
2. Fatty or Malabsorptive Diarrhea
Malabsorptive diarrhea occurs when the digestion or absorption of nutrients is impaired. It often includes the presence of excess fat in the stool (appearing as frothy or greasy stool), a foul odor to the stool, and symptoms such as bloating, gas, and weight loss.
Malabsorptive diarrhea improves with fasting.
3. Inflammatory or Exudative Diarrhea
This type of diarrhea results from a damaged intestinal lining. Inflammation is usually present in the bowels, which is why it is described as inflammatory. There is often blood or pus present in the stool so it is sometimes referred to as exudative diarrhea.
腹泻的类型
腹泻有三种基本类型:水样腹泻、脂肪性腹泻(吸收不良)和炎症性腹泻。并不是所有的腹泻都是水样的,吸收不良的,或者是炎症性的,因为有些类型是重叠的。此外,个体可能经历腹泻的多种原因,因此呈现更复杂的情况。
1. 水样腹泻
水样腹泻的特征是肠流或水样运动。水样腹泻可分为渗透性、分泌性和功能性三大类。
渗透性腹泻
当某些东西从身体的其他部分吸取水分进入肠道时,就会发生渗透性腹泻。在大多数情况下,当一个人停止摄入导致水进入肠道的食物或物质时,渗透性腹泻就会停止。
分泌性腹泻
分泌性腹泻包括大便量增加,腹泻通常发生在整晚,即使禁食也会持续。
分泌性腹泻发生时,更多的水被释放到肠道比平时。这是由于增加分泌或减少吸收的电解质在肠道。造成这种混乱的原因有很多。
功能性腹泻
功能性腹泻包括胃肠道肌肉过度运动,以腹泻量较小为特征。功能性腹泻在夜间和禁食时有所改善。功能性腹泻没有任何相关的腹痛。
患有功能性腹泻的人通常不会一直有这种症状。正常的肠道运动,甚至便秘也可能发生在腹泻之间。
功能性腹泻病因不明。这种类型的腹泻没有结构或生化异常,只是部分胃肠道系统功能不太正常。
2. 脂肪性或吸收不良的腹泻
当营养物质的消化或吸收受到损害时,就会发生吸收不良腹泻。它通常包括粪便中的多余脂肪(表现为泡沫或油腻的粪便),粪便的恶臭,以及诸如腹胀、胀气和体重减轻等症状。
禁食可改善吸收不良腹泻。
3.炎性或渗出性腹泻
这种类型的腹泻是由肠道内壁受损引起的。炎症通常出现在肠道,这就是为什么它被称为炎症。粪便中经常有血或脓,所以有时被称为渗出性腹泻。
Digestive System Disorders:
Dietary Intolerance – Conditions such as celiac disease (gluten intolerance), lactose intolerance, and fructose intolerance are caused by enzyme deficiencies or abnormalities in the intestinal mucosa, this impairs the necessary functions of the GI tract and leads to malabsorption of nutrients. When nutrients are not absorbed properly, they build up in the intestine and draw fluids to the intestines. Such conditions can cause secretory or malabsorptive diarrhea.
Bile Acid Deficiency – A reduced amount of bile acids will impair the absorption of fats resulting in fatty or malabsorptive diarrhea. Low bile acids can be due to a number of causes including decreased liver or gall bladder function, low cortisol levels, or prescription medications. Bile acid deficiency is different than bile acid malabsorption, which involves excess secretion of bile acids into the bowels, resulting in secretory diarrhea.
Pancreatic Insufficiency – The loss of pancreatic secretions which occurs in cystic fibrosis and pancreatitis also effect digestion and absorption of nutrients.
Irritable Bowel Syndrome (IBS) – Functional diarrhea is one of the symptoms experienced by people with IBS. Functional diarrhea and IBS are often mistaken for the same condition, but they are not since IBS includes other symptoms, such as abdominal pain.
Inflammatory Bowel Diseases – There is usually a lot of damage to the intestinal wall in inflammatory conditions such as Chron’s disease, ulcerative colitis, and diverticulitis. Exudative diarrhea is most common in such conditions. Secretory diarrhea occurs in microscopic colitis and in earlier stages of some of the other inflammatory bowel diseases.
消化系统导致的腹泻:
饮食不耐症——像乳糜泻(谷蛋白不耐症)、乳糖不耐症和果糖不耐症都是由肠道粘膜的酶缺乏或异常引起的,这损害了胃肠道的必要功能,导致营养物质的吸收不良。当营养物质没有被正确地吸收时,它们就会在肠道中堆积,并将液体吸入肠道。这种情况会导致分泌性或吸收不良的腹泻。
胆汁酸不足-胆汁酸的减少会损害脂肪的吸收,导致脂肪性或吸收不良的腹泻。胆汁酸低的原因有很多,包括肝脏或胆囊功能下降,皮质醇水平低,或处方药。胆汁酸缺乏不同于胆汁酸吸收不良,后者是胆汁酸分泌过多进入肠道,导致分泌性腹泻。
胰腺功能不全-囊性纤维化和胰腺炎导致的胰腺分泌减少也会影响营养物质的消化和吸收。
肠易激综合征(IBS) -功能性腹泻是IBS患者经历的症状之一。功能性腹泻和肠易激综合症经常被误认为是同一种疾病,但实际上并非如此,因为肠易激综合症还包括其他症状,如腹痛。
炎症性肠病——在炎症状态下,如慢性疾病、溃疡性结肠炎和憩室炎,通常会对肠壁造成很大损害。在这种情况下,渗出性腹泻最常见。分泌性腹泻发生在显微镜下结肠炎和一些其他炎症性肠病的早期阶段。https://flowingfree.org/diarrhea-no-more-leave-the-toilet-behind/
https://www.ibstales.com/caltrate-plus.htm
Ingredients in Caltrate plus
哈佛医学院 哈佛干细胞研究院 2017
了解肠道是如何替换和修复自身的
哈佛大学的研究人员发现,在小鼠肠道中,成熟细胞可以恢复为干细胞,以促进组织再生和修复
哈佛干细胞研究所、达纳-法伯癌症研究所和哈佛医学院的研究人员发现了一种之前未知的机制,它在肠道内层再生中发挥重要作用。他们的发现为这个每天都在发生变化的组织如何维持自身提供了新的见解。
小肠是人体中再生能力最强的器官,每5到7天再生一次叫做上皮的内膜。细胞的持续更新使上皮细胞能够承受在分解食物、吸收营养和消除废物的过程中所遭受的持续磨损。
在一项细胞干细胞研究中,研究人员发现成熟细胞,而不是其他干细胞,负责补充小鼠肠道隐窝中干细胞的数量。
“这是一项非常基本的发现,它让我们能够深入了解组织是如何组织起来的,”哈佛干细胞研究所(Harvard Stem Cell Institute)的首席教员、丹那-法伯癌症研究所(Dana-Farber Cancer Institute)和哈佛医学院(Harvard Medical School)的医学教授、该研究的资深作者拉梅什·希夫达萨尼(Ramesh Shivdasani)说。
在过去的10年里,科学家们一直认为,肠道上皮细胞再生的程度取决于另一批干细胞的存在,这些干细胞在需要的时候仍然处于休眠状态。相反,根据这项新研究,当工作干细胞被耗尽时,一些类型的成熟细胞在经历了一个叫做去分化的过程后会将自己转化为干细胞。
“这个过程是可能的,因为我们体内所有的细胞都有相同的遗传密码,”Shivdasani说,“但是使每个细胞不同的是那些实际活跃的遗传密码的部分。”
要想发生去分化(De-differentiation),细胞需要重新排列其DNA在细胞核内折叠成染色质的方式。这将改变哪些基因是活跃的,这通常被认为是不可能发生的。
然而,在耗竭了小鼠肠道隐窝中的原始干细胞后,Shivdasani和他的同事们分析了每个细胞类型特有的分子标记和染色质签名,以确定存在哪些类型的细胞和数量。
他们发现,随着新干细胞的出现,两种不同类型的细胞群发生了变化,这表明这些细胞正在去分化并成为干细胞。所有证据都表明,同样的机制也可能发生在人类身上。
“肠道似乎具有巨大的可塑性,”Shivdasani说,“在数以千计的肠隐窝中,我们可以看到染色质的展开。”
这项研究揭示了发生在我们组织深处的基本过程,使我们的身体能够处理损伤,并可能为未来确定治疗靶点提供基础。然而,Shivdasani指出,要了解这些知识是如何引导对特定疾病的治疗还为时过早。
“我们仍然不明白肠内的成熟细胞是如何知道干细胞缺失的,”Shivdasani说,但“我们已经表明,染色质屏障是容易可逆的,正在进行去分化的细胞可以被捕获和研究,这是一个重要的开始。”
这项研究得到了国家糖尿病、消化和肾脏疾病研究所、国家过敏和感染疾病研究所、国家卫生研究所的资助,以及神经内分泌肿瘤研究基金会和泛大众挑战组织的捐赠。
赞叹~生命神奇的自愈力; 中医 "脾胃为后天之本”的远见;
启发~既然成熟的肠道上皮细胞可以“去分化”返祖为干细胞,正常细胞的癌变和普通癌细胞返祖为“癌症干细胞”,也就不难理解了。
Understanding how the intestine replaces and repairs itself – Harvard Gazette
https://news.harvard.edu/gazette/story/2017/07/understanding-how-the-intestine-replaces-and-repairs-itself/
Autism and the Human Gut Flora
Previous research into autism has raised the possibility that a link exists between the disorder and the human gut microflora. Research has remained limited due to the unwillingness of the orthodox medical establishment to adopt treatments suggested by this research. This short review aims to summarise the research completed to date and evaluate the relevance of a link between autism and a possible imbalance in the human gut microflora. It appears that yeasts (Candida species in particular) and clostridia may play an important role in the development of autistic symptoms. It is suggested that the control of the growth of these species may reduce the severity of autistic symptoms but is unlikely to offer a cure.
Introduction
Previous research into autism has raised the possibility that a link exists between the disorder and the human gut microflora. However the relevance of this to sufferers of Autism has remained somewhat un-researched. As a consequence, it appears that the treatments suggested previously have remained unused and regarded by the orthodox medical establishment as irrelevant. This short review will consider the limited research completed to date.
Autism
The term autism is usually associated with the syndrome first described by Kanner (1943). In more recent years specific criteria have been set out to aid in the diagnosis of the disorder (Shaw et al, 1999). Autism typically develops early in childhood, however causality, explanation and treatment are often hotly debated. Symptoms can include (but not necessarily), hyperactivity, loss of eye contact, decreased vocalisation (i.e. loss of language), stereotypical behaviours, poor academic performance and other similar social deficits. Other similar disorders exist. These include Asperger Syndrome, Attention Deficit Hyperactivity Disorder (ADHD), Pervasive developmental disorder (PDD) and many others, where symptoms are similar to Autism but specific differences are demonstrated.
Yeast metabolites in the Urine of Autistic Children
Yeasts constitute only a very small proportion of the population of the gut (Holzapfel et al, 1998) under normal conditions – possibly kept from growing via competition from bacterial species and certain immune functions. However, Shaw et al (1999) has proposed that Autism (or at least many of the symptoms) may be a consequence of an overgrowth of candida species and a selective IgA deficiency. Following treatment with antifungal drugs and a gluten and casein free diet, a child rated as having severe autism improved such an amount as to be classed as a higher functioning individual with autism.*
It has been shown previously that children exhibiting autistic features have increased excretion of arabinose and the analogs of Krebs Cycle metabolites (including tartaric acid) (Shaw, Kassen & Chaves, 1995). Using Gas Chromatography/Mass Spectrometry (GC/MS) to test for urinary metabolites, it was found that the children had extremely high values of tartaric acid. The only source of tartaric acid is yeast (Shaw, 1999). Many reports have suggested that the onset of autism may be related to the occurrence in children of otitis media (Kontstantareas & Homatidis, 1987). It is common to treat otitis media with some sort of broad-spectrum antibiotic. Intestinal overgrowth of yeast and certain anaerobic bacteria are a well documented outcome of the administration of broad spectrum antibiotics (Kennedy & Volz, 1983; Danna et al, 1991; Ostfield et al, 1977; Kinsman et al, 1989; Van der Waaij, 1987; Samsonis et al, 1993, 1994a,b).
To evaluate this, it is known that Shaw (1999, 1996) has used GC/MS to test the urinary content of metabolites following the administration of Nystatin, an antifungal drug. Accordingly it was found that urinary tartaric acid declined to zero after about 60 days. When the Nystatin dose was cut to half, levels appeared to rise. Associated with this was seen an improvement in eye contact, a reduction in hyperactivity and an improvement in sleep patterns. It is unclear as to the method used, however these are interesting results. In other work, Shaw (1999) has evaluated the progress of the Herxheimer reaction of yeast die off. Values for the microbial metabolites described above increased dramatically during the first three days and began to normalise near day four. It is unclear as to the relevance of the point that many children take antibiotics for a whole series of illnesses, but do not develop autism.
Gupta et al (1996) have studied some possible modulating factors in Autism. These include immunodeficiencies and probable differences in biochemical detoxication factors which appear to be very common in autism,. It has been estimated that a high percentage of autisitic children have a significant immune dysfunction that may include myeloperoxidase deficiency, a genetic deficiency that impairs the action of white blood cells on yeast cells, IgA deficiency, complement C4b deficiency, IgG deficiency or IgG subclass deficiency (Gupta et al, 1996). In the above study, a complete remission of autisitic symptoms was achieved by infusions of gamma globulin in one case. Shaw (1999) has suggested that it seems increasingly likely that the immune system takes an inventory of bacteria and yeast cells present in the gut soon after birth. This inventory is performed by CD5+ B-cells. These cells may play a role in regulating the secretion of IgA, the antibody class that is secreted into the intestinal tract and which may select which microorganisms are tolerated in the gut. Continuing, it is suggested that the eradication of the normal gut flora when antibiotics are administered repeatedly during infancy may cause the CD5+ cells to reject normal cells and award immune tolerance to species that potentially could cause harm. Either antibiotic use in infancy or yeast infection of the mother during pregnancy may result in later tolerance to yeast. This would possibly extend any remission from symptoms induced by methods of treatment suggested for autism.
Yeast and Tartaric Acid Toxicity
Shaw, Kassen & Chaves (1995) noted in their initial study of the two brothers with autism, that in addition to their autistic characteristics, they exhibited severe muscle weakness. Both of these brothers excreted large amounts of tartaric acid in their urine. It is known that tartaric acid is an analog of malic acid. Malic acid is a key intermediate in the Krebs cycle which is responsible for the extraction of most of the energy from food substrates. Tartaric acid is presumably seen as toxic since it would inhibit this pathway and limit energy production. This may be the reason for the muscle weakness seen in the two autistic brothers. It has also been shown that Candida albicans produce gliotoxins (Shah and Larsen, 1991 and 1992) and immunotoxins (Podzorski et al, 1989; Witken, 1985), which may further impair the immune system. This would have relevance in terms of promoting yeast growth and increasing the chances of additional infections from bacteria leading to antibiotic usage again.
Arabinose, Yeast and Autism
Shaw, Kassen & Chaves (1995), also identified high levels of arabinose in the urine of the autistic children. The exact biochemical role of arabinose is unknown, but a closely related yeast alcohol, arabitol has been used as a biochemical indicator of invasive candidiasis (Kiehn et al, 1979; Wong et al, 1990, Roboz & Katz, 1992). It is thought that arabitol produced by the yeast in the gut is absorbed into the portal circulation and then converted to arabinose by the liver. Elevated protein-bound arabinose has been found in the serum glycoproteins of schizophrenics (Varma & Hoshino, 1980) and in children with conduct disorders (Varma et al, 1983).
Arabinose reacts with the epsilon amino group of lysine in a wide variety of proteins and may then form cross-links with arginine residues in an adjoining protein (Sell & Monnier, 1989). Arabinose has therefore been implicated in protein modification via cross-linking the proteins and altering both biological structures and functions of a wide variety of proteins. Shaw et al (1999) has proposed that this may include proteins involved in the interconnection of neurons. Decreased clinical symptoms of autism after antifungal treatment might be due to decreased arabinose and pentosidine formation (cross-linked proteins described above). This would result in fewer random neural connections and increased numbers of neural connections that are oriented to the child’s environment. The influence of a number of vitamins on candida activity and the operation of many metabolites of candida has been discussed (Mahler & Cordes, 1966). It is commonly found that children with autism experience improved symptoms following removal of gluten and casein from the diet (Shattock et al 1990, 1991, 1996, 1997; Reichelt et al, 1981, 1986; Knivsberg et al, 1990). It is unclear whether there is a link with arabinose and pentosidine formation, however given the wide distribution of these proteins in foods, there may be a relevant process involved with respect to autistic symptoms. Research into this may be useful.
Clostridia and Autism
Bolte (1998) outlined the possibility of a subacute, chronic tetanus infection of the gut as the underlying cause for symptoms of autism observed in some individuals. Here it is postulated that a percentage of individuals with autism have a history of extensive antibiotic use. As above it is known that oral antibiotics significantly disrupt protective gut microflora, creating a favourable environment for colonisation by opportunistic pathogens. Clostridium tetani is a ubiquitous anaerobic bacillus that is known to produce a potent neurotoxin. The normal site of binding for tetanus neurotoxin is the spinal cord, yet the vagus nerve is capable of transporting tetanus neurotoxin, providing a route of ascent from the intestinal tract to the central nervous system and bypassing the spinal cord. The result would be the typical symptoms of a tetanus infection would not be evident. Once in the brain the tetanus neurotoxin disrupts the release of neurotransmitters. This may explain the wide variety of behavioural deficits apparent in autism. Bolte (1998) presents evidence of lab animals exhibiting many of these behaviours after being injected in the brain with tetanus neurotoxin, and also of children with autism showing a significant reduction in sterotyped behaviour following treatment with antimicrobials effective against intestinal clostridia.
Shaw (1999) has developed a similar technique to yeast metabolite analysis by GC/MS for clostridia metabolites. While it has not been fully identified it has been shown that tyrosine derivative, a compound very similar but not identical to 3,4-dihydroyphenylpropionic acid is raised in the urine of children with various conduct disorders. It has also been pointed out that tyrosine, a substrate used by the body for the production of neurotransmitters, might imply that this unidentified product is important in altering key biochemical pathways for neurotransmitters in the brain. The relevance to autism remains unclear. Clearly a treatment to reduce the numbers of clostridia in the gut might help to reduce the effects of this inhabitation. However, clostridia are spore forming meaning recolonisation is possible even after treatment. The relevance of probiotic and prebiotic treatments has not been researched, yet this appears a reasonable approach to aid suppression of the clostridia species.
Human Gut Flora and Gluten and Casein Intolerance in Autistic Children
Yeasts, including Candida albicans are known to secrete a number of enzymes. These may include phospholipase, which will break down phopholipids and proteases such as secretory aspartate protease which break down proteins. These enzymes may partially digest the gut membranes and lining itself. Furthermore, it is known that the mycelium and chlamydospore are capable of tissue invasion (Nolting et al, 1994). It is likely that such factors could increase the permeability of the gut. This has important consequences in terms of food absorption and digestion. In terms of autism symptoms this may be highly relevant. It has been shown that incompletely broken down portions of gluten and casein may be crossing the gut into the blood and having an opioid effect in autistic children. Their symptoms being a consequence of this opioid action (Reichelt, 1981; Shattock et al, 1990). While many mechanisms have been suggested for this incomplete breakdown, it seems key that a yeast and/or clostridia overgrowth would affect this in some way.
Conclusions
Products of the human gut microflora in relation to autism and its symptoms appear to have been largely ignored in the past. However they appear to be relevant certainly in terms of yeast and clostridia species. Abnormal bacterial metabolites of tyrosine appear to be elevated in autism and this seems related largely to an overgrowth in clostridia species. Treatments to control this have resulted in significant clinical improvement or complete remission of symptoms in some cases. The issue of whether probiotic and prebiotic treatments are relevant, needs to be researched. Elevation of yeast metabolites such as tartaric acid and arabinose appears to be even more common in autism. The arabinose appears to be involved in abnormal protein binding which may adversely affect neuron connection and this may have relevance to the appearance of autistic symptoms. Given the intolerance autistic children appear to have to gluten and casein, an involvement of arabinose may be important. However, the exact biochemical role of arabinose remains unclear. Tartaric acid from the yeast overgrowth may have a direct toxic effect on the muscles and is a key inhibitor of the Krebs Cycle that supplies raw materials for gluconeogenesis. The implications of this appear to be detrimental to autistic function.
While it is clear that abnormal metabolites from the human gut flora may contribute to autistic symptoms, it is certain that many other systems and pathways are involved. It is possible that many are functioning simultaneously but at varying levels between individuals. This may give rise to the differences in symptoms exhibited by sufferers. This may also explain the similarity of autism and other chronic behavioural disorders such as Asperger Syndrome, PDD, ADHD, ADD and Rett syndrome. While other pathways may exist, research into the role of human gut flora and the full identification of species involved may allow for more directed treatments. While it will not cure the disorder, modifications of gut flora function might improve symptoms significantly.
* The Childhood Autism Rating Scale, an observational measure of various aspects of autism, for the child in question decreased from 43 (severely autistic) prior to introduction of these therapies to a value of 29 (non-autistic) after therapy.Autism and the Human Gut Flora
https://www.ei-resource.org/articles/autism-articles/autism-and-the-human-gut-flora/?tmpl=component
.gif)
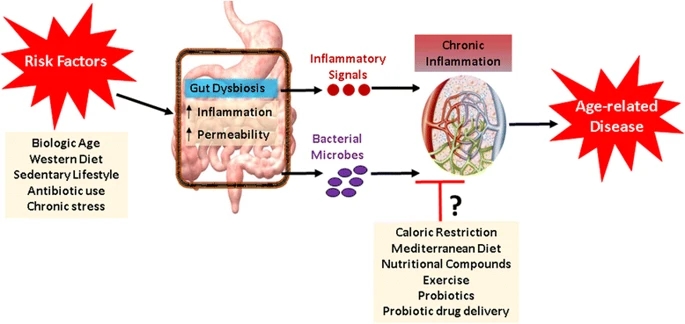
.png)

.png)