﹛
Effect of renin每angiotensin system on senescence - Mogi ...
https://onlinelibrary.wiley.com/doi/10.1111/ggi.13927Apr 29, 2020 ﹞ Brain Ang II participates not only in regulating blood pressure, sympathetic activity, vasopressin secretion, thirst and sodium appetite, 41 but also in the pathophysiology of central nervous system diseases such as stroke and neurodegenerative diseases.
Your heart is constantly working, pumping about 2,000 gallons of blood a day. By staying hydrated 每 i.e. drinking more water than you are losing 每 you are helping your heart do its job. A hydrated heart is able to pump blood more easily, allowing the muscles in your body to work even better.
RELATED: Hydration 101: What You Need to Know
Dehydration causes strain on your heart. The amount of blood circulating through your body, or blood volume, decreases when you are dehydrated. To compensate, your heart beats faster, increasing your heart rate and causing you to feel palpitations. Also your blood retains more sodium, making it tougher for it to circulate through your body.The Importance of Hydration for Your Heart | UPMC HealthBeat
https://share.upmc.com/2014/09/importance-hydration-heart/﹛
﹛
Dehydration : Sudden, silent, and very serious
﹛
With all the talk of global water shortages, it*s amazing how much water is actually on the planet 每 including our own human bodies. More than half of the human body is made up water 每 in fact, as newborns, we*re mostly water! Water is vital to the human body 每 without it, we die. We need it to nourish us, to cool us, to hydrate us.
It would seem that with all that water in us, we*d never go thirsty, but we use water constantly 每 when we breathe, when we move,when we urinate, when we sweat, when we sleep. We use up water simply by existing. And we use up a lot of it. We need to replenish our supply constantly. Our bodies are pretty good at letting us know when we need to replenish our supply of the good stuff. We get pangs of thirst that signify the need to refuel and those hunger pangs sometimes aren*t really 每 they are thirst pangs disguised as a need for food. We need at least eight glasses a day, but this will also depend on the size of a person, their health and their activity levels. Another way to gauge water needs is to drink half your body weight in ounces. In other words, if you weight 150 lbs, you will need at least 75 oz of water every day. It doesn*t always have to be plain water 每 liquids like tea or coffee also count, although liquids like alcohol actually dehydrate and cannot be considered a source of hydration.
Dehydration is a problem for many, and sometimes we don*t even realize it until major damage has been done. We*ve heard stories of young sports players dropping dead on the field from heat exhaustion and dehydration, and of older people collapsing in a heat wave. Anyone involved in strenuous activities needs to make sure their water intake is higher than their output. Children playing outside need to be continually hydrated and persons who work outside all day 每 especially in the summer months, need to take great care to make sure they drink plenty of water. And those of us who have diabetes, or have an illness where diarrhea or vomiting is present, are at risk.
So what happens when we don*t drink enough? Sometimes a glass or two is all we need to replenish our systems. But sometimes there*s a real danger of collapse, of worse 每 when our body lacks the water it needs.
Our body will preserve as much liquid as it can, so our mouths and eyes will become dry and nausea may set in. We may become dizzy, light headed, and confused. Our urine will decrease and darken in color and our eyes may yellow. Someone with these symptoms needs immediate medical attention, especially a very young child or older person.
But healthy adults can also suffer the perils of dehydration, no matter how healthy they think they are. Without adequate water, it doesn*t take long for the body to show the ill-effects. And left untreated, it can cause kidney failure, brain swelling, coma, or hypovolemic shock. This kind of shock can cause death to happen quickly, if left untreated. People from over-exerted athletes, dancers and entertainers to under hydrated kids and grandparents, can suffer this kind of shock and it*s fast and furious. Champion mixed martial arts fighter Gina Joy Carano was rushed to hospital with dehydration in 2007 and Houston Astros reliever Wesley Wright was also rushed to hospital two weeks ago, apparently suffering from the same ailment. Dehydration was a constant worry for recently deceased singer Michael Jackson who lost up to 10 lbs from exertion and heat during every concert, rehearsed constantly for his planned concert comeback, and had trouble keeping his body healthy due to a poor diet and possible drug use/abuse.
How do we avoid dehydration? By not using more water than we take in and by limiting alcohol and drugs, as well as avoiding situations of over-exertion and heat exposure. Sufferers from illnesses that cause vomiting or diarrhea, as well as conditions like diabetes need to make sure they monitor their water intake. The very young and old need to be cared for when ill or during the long summer months.
Because dehydration can be sudden - and silent 每 it can also be deadly. If it doubt, or when symptoms are more than just a moderate thirst or sweat, care needs to be taken to rehydrate directly. And if rehydration does not happen quickly, seek medical attention immediately.
Tell Us
Did you know that dehydration can cause death? Have you experienced dehydration? How did you replenish your water supplies?Dehydration : Sudden, silent, and very serious - Wellness Info and Tips
https://www.empowher.com/community/share/dehydration-sudden-silent-and-very-serious﹛
CHRONIC DEHYDRATION Part 10: It Triggers Breathing ...
https://hbmag.com/chronic-dehydration-part-10-it-triggers-breathing-problems
Jun 01, 2013 ﹞ Breathing problems like allergies, asthma, and COPD are due to dehydrated tissues that become more sensitive. Water is used in the nasal passages, bronchial tubes, and lungs and to keep them moist. But every breath outward expels moisture from these tissues, and every breath in brings drying air. Under hydrated conditions water is rapidly replaced.﹛
The Importance of Staying Hydrated if you have COPD
https://www.1stclassmed.com/blog/the-importance-of-staying-hydrated-if-you-have-copd
Jul 11, 2017 ﹞ Dehydration could be the cause for asthma attacks or COPD exacerbation following eating. Water is key to the digestive process. It helps break down food and if water isn*t available, it is taken from vital organs. We*ve already learned that lungs are 83% water so #﹛
Cell Mol Life Sci. Author manuscript; available in PMC 2016 Oct 1.
Airway Hydration and COPD
Arunava Ghosh, R.C. Boucher, and Robert Tarran
Cystic Fibrosis Center/Marsico Lung Institute and the Department of Cell Biology and Physiology, 7102 Marsico Hall, The University of North Carolina, Chapel Hill, NC, 27599-7248
Abstract
Chronic obstructive pulmonary disease (COPD) is one of the prevalent causes of worldwide mortality and encompasses two major clinical phenotypes, i.e., chronic bronchitis (CB) and emphysema. The most common cause of COPD is chronic tobacco inhalation. Research focused on the chronic bronchitic phenotype of COPD has identified several pathological processes that drive disease initiation and progression. For example, the lung*s mucociliary clearance (MCC) system performs the critical task of clearing inhaled pathogens and toxic materials from the lung. MCC efficiency is dependent on: (i) the ability of apical plasma membrane ion channels such as the cystic fibrosis transmembrane conductance regulator (CFTR) and the epithelial Na+ channel (ENaC) to maintain airway hydration; (ii) ciliary beating; and, (iii) appropriate rates of mucin secretion. Each of these components is impaired in CB and likely contributes to the mucus stasis/accumulation seen in CB patients. This review highlights the cellular components responsible for maintaining MCC and how this process is disrupted following tobacco exposure and with CB. We shall also discuss existing therapeutic strategies for the treatment of chronic bronchitis and how components of the MCC can be used as biomarkers for the evaluation of tobacco or tobacco-like-product exposure.
Keywords: Airway surface liquid, Cystic Fibrosis, CFTR, ENaC, Mucus, Tobacco smoke
Introduction
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death worldwide and is projected to become the third leading cause of death by 2030 [1]. COPD is characterized by a persistent airflow limitation that is progressive and associated with an augmented chronic inflammatory state [2]. COPD occurs after chronic environmental exposure to tobacco smoke and/or other noxious particles or gases [2, 3], but may also have a genetic component that predisposes certain populations to COPD [4].
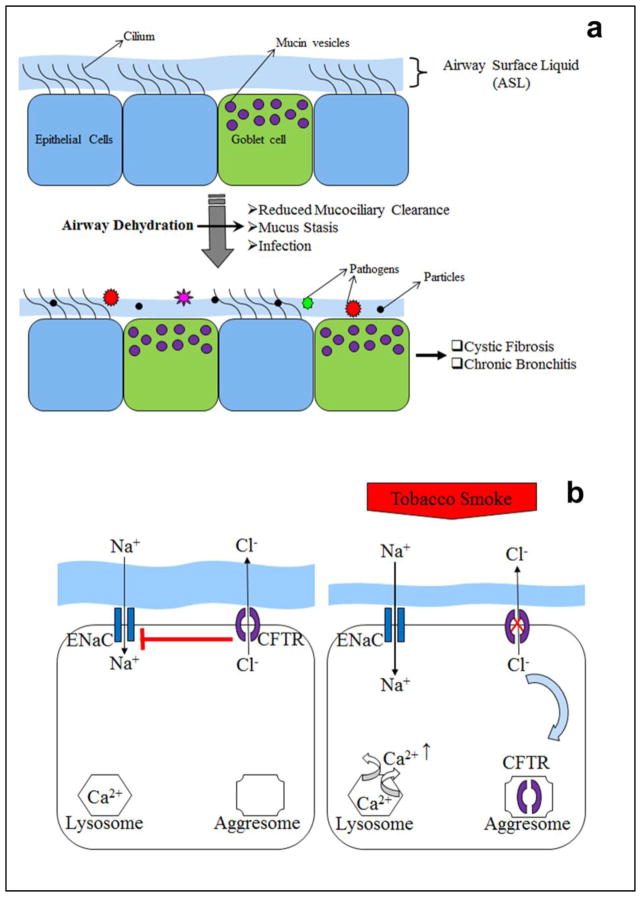
Chronic bronchitis (CB) and emphysema are considered as the two major clinical and epidemiological phenotypes of COPD. Production of sputum and cough for at least 3 months over two consecutive years defines CB, whereas emphysema is defined by the ※destruction of the gas-exchanging surfaces of the lung§ [2]. Most COPD patients exhibit symptoms of both emphysema and CB [5]. However, CB is typically the more prevalent phenotype [6]. The clinical manifestations of CB (sputum production and a failure of mucus transport concomitant with chronic inflammation) are similar to the symptoms reported in early cystic fibrosis (CF) lung disease. CF is caused by mutations in the CFTR anion channel [7], which lead to reduced airway surface liquid (ASL) volume and subsequent mucus dehydration, mucus stasis and recurring airway infections [8每10]. Although the causal factors appear different, recent evidence suggests that the acquired dysfunction of CFTR-mediated ion transport following chronic tobacco exposure may also contribute to CB pathogenesis [11]. Indeed, the mucus hydration status of the airways has both direct and indirect effects on pulmonary health [12, 13]. Thus, it has been proposed that the pathophysiology of the two diseases, i.e. CF and COPD, share similar initiation and progression scenarios, with compromised mucus hydration being common [14, 15]. In this review, we shall discuss the pathophysiology of the chronic bronchitis form of COPD (see Figure 1), and how this knowledge may generate (i) novel treatment regimens and (ii) clinically relevant novel biomarkers of exposure.
A, The compromised airway hydration status leads to defective mucociliary clearance leading to goblet cell hyperplasia, mucus stasis and infection with subsequent manifestations of diseases like cystic fibrosis and chronic bronchitis. B, Exposure to tobacco smoke leads to relocation of surface CFTR to aggresome and release of Ca2+ from the lysosome resulting in augmented absorption of Na+ through ENaC and ASL height decrease.
﹛
Causes of COPD
Mortality in COPD in part reflects pulmonary failure, but may be dominated by comorbidities such as cardiovascular disease and lung cancer. Although the principal etiological factor for COPD is chronic tobacco smoke exposure, the involvement of genetic, epigenetic and host-factors is evident by the fact that only a fraction of smokers develop the disease. The CB incidence rate in smokers is typically suggested to be around 25% [16]. However, COPD may be underreported, and in the USA, for example, ~12 million adults have been diagnosed with the disease, but an equal number of people likely suffer from COPD without an official diagnosis. [17]. In addition to tobacco exposure, both indoor and outdoor pollution can also cause COPD in non-smokers [18每21]. As such, biomass smoke exposure (e.g. smoke from wood burning stoves) and occupational pollutant exposure are also considered significant risk factors for COPD [22, 23].
Apart from external etiological factors, the most crucial genetic risk factor for COPD is 汐1-anti trypsin deficiency [24]. The 汐1-anti trypsin protein is coded by the serpin peptidase inhibitor, clade A, member 1 (SERPINA1) gene [25]. 汐1-anti trypsin deficiency is an autosomal co-dominant state that leads to reduced levels of 汐1-anti trypsin in circulating blood and corresponding failure to inhibit neutrophil elastase [26]. Even without exposure to tobacco smoke, the subsequent uncontrolled neutrophil elastase activity can lead to lung damage and early onset COPD [27]. Significant pulmonary destruction is visible by the age of 30 in many patients [27], and 汐1-anti trypsin deficiency-induced lung disease worsens upon exposure to environmental toxicants such as tobacco smoke [4, 26, 28, 29].
The airway surface mediates normal mucus clearance and reduced ASL volume, i.e. hydration, produces the reduction in mucus clearance. Severe reductions in mucus clearance are associated with increase mucus stasis/mucus adhesion, which lead to the increase in inflammation and bacterial infections characteristic of CF and CB [30]. Investigations into the pathophysiologic basis of chronic bacterial airways infection typically reveal that airways obstruction is the primary cause of this syndrome [31, 32]. Airways obstruction can be produced by intraluminal airway tumors and inhalation of foreign bodies. However, the most common form of airways infection is associated with the intraluminal obstruction produced by mucus adhesion, mucus plaques, and ultimately, mucus plugs [33每35]. For example, the pathologic studies of Hogg et al, indicated that this scenario is central to the pathogenesis and progression of the CB phenotype of COPD [36, 37].
Go to:
The mucus clearance component of the lung*s innate defense system
The lung is frequently exposed to inhaled pathogens and pollutants. However, by the time that inhaled air reaches the alveolar surfaces, it has been filtered and humidified [38]. These functions are largely performed by the innate defense system of the upper respiratory tract [38, 39]. Under normal conditions, pulmonary secretions contain soluble anti-microbial factors, macrophages, and mucins that each play a role in mediating the initial responses to toxic or infective inhalations [40每42]. The surface epithelium of the airways coordinates these responses via multiple specialized cell types, including columnar ciliated cells and mucous (goblet) cells, along with underlying basal cells. Mucins were secreted by the surface epithelial goblet cells in parallel with mucins derived from submucosal glands, which in the large airways open onto the airway surface through a series of ducts [43].
Most mucosal surfaces that interface with the outside world are ※wet§ mucosal surfaces [44]. As a general principle, ※wetness§ is a required characteristic for mechanical clearance, e.g., is required for the efficiency of blinking, swallowing, and indeed, mucus clearance [45]. Heretofore, it was generally assumed that the major determinants of the efficiency of mucus clearance were the actions of cilia and the rate of mucin secretion [46, 47]. Indeed, both are important contributors to the overall efficiency of mucus clearance, but evidence now suggests that the hydration status of the environment is the dominant variable for the efficiency of the mucus clearance process [48]. Under physiologic conditions, the mucus layer acts as a reservoir for water, i.e., it can donate or accept added liquid, to maintain apposition of the inner surface of the mucus layer with the tips of the cilia [48]. This regulation serves to preserve the hydration status of the pericilliary liquid layer (PCL), which is required for cell surface lubrication and for efficient ciliary beating. Mucus clearance can accelerate above basal rates when liquid is added to the airway lumen, consistent with the in vitro studies of human bronchial epithelial cultures (HBECs) with confocal and epifluorescence microscopy [48, 49]. When liquid is removed from the ASL in an inappropriate fashion, e.g., as in CF and CB [15], the supply of water ※donated§ from the mucus layer to the PCL can be exhausted, and the PCL will become dehydrated and collapse. The thickened (concentrated) mucus layer then comes into contact with cell surface microvilli, cilia and ultimately adheres (14). As a result of this interaction, mucus clearance ceases and the nidus for plaque/plug formation is generated.
Efficient mucus clearance from airway surfaces requires the coordinated activities of a ※transported layer§, i.e., the mucus layer, a cell surface layer, and cilia [50, 51]. The biophysical requirements for a mucus layer to exhibit efficient clearance are daunting and only beginning to be understood. The mucus layer must have viscoelastic properties that enable the transfer of energy from ciliary beating to the vectorial movement of mucus towards the mouth [45]. The mucus layer must also have properties that will allow it to bind and entrap virtually all deposited particles so they may be removed from the pulmonary surface, yet not adhere to the cell surface [52]. Finally, the mucus layer must be able to adapt to local stresses to maintain its viscoelastic and other properties to provide optimum transport [44, 53, 54].
A new concept has emerged as to how the mucus layer is organized to provide these functions [55]. High molecular weight, extraordinarily long (0.5每20 米m) mucins (MUC5AC, MUC5B) are secreted onto the airway surface to form the mucus layer. However, the functional properties of this layer are also provided by interacting globular proteins. Indeed, this mucin interactome may provide both cross-links between mucin monomers and serve to build complex molecular compartments with their own innate defense activities [56, 57]. In parallel, our concept of the cell surface layer, i.e. the PCL, has undergone a dramatic evolution within the past several years. The emerging data on the identification of cell surface tethered mucins (MUC1, 4, 16) led to the hypothesis that the periciliary environment is comprised of a polyelectrolyte gel rather than a liquid layer [57]. This concept is important because it predicts that the distribution of water between the gels on the airway surface will in part reflect the relative concentration of mucus in each compartment. The ※water drawing§ power of each gel can be measured in terms of its osmotic modulus or pressure [58]. Importantly, mucin concentrations in diseased lungs cannot accurately be measured using Western blot-based approaches and instead must be measured by physic-chemical techniques. As a case in point, many of the epitopes in the mucins found in CF mucus that are recognized by antibodies are degraded in the highly proteolytic environment of the CF lung, yielding erroneous results by Western blot. However, both mass spectrometry analysis and refractometry measurements of the void volume indicated that CF mucin levels are increased relative to normal mucinlevels [58].
Go to:
Goblet cells and mucins of the conducting airways
Chronic mucus hypersecretion has been identified as a potential risk factor for mortality in COPD because of the associated accelerated loss of lung function with this exposure [59]. One of the characteristic features of obstructive lung diseases is mucus hypersecretion that is driven in part by glandular hypertrophy but also by goblet cell hyperplasia and metaplasia, especially in the glandless small airways. As such, goblet cells contribute to the hypersecretion of mucins in CB airways [60]. The replacement of ciliated epithelial cells by goblet cells is complex and is in part driven by inhibition of apoptosis by EGFR, and/or IL-13-dependent activation of MEK/ERK [61每63]. The increase in mucin secretion in CB lungs may provide a protective coat of mucus that line the airways to limit toxicant exposure. Accordingly, while mucus obstruction is deleterious in the long term, in the short term, it may protect the airways against tobacco exposure by acting as a barrier to tobacco smoke diffusion. Mucin knock out mice exist (MUC5b−/−) and exhibit increased susceptibility to infection [64], but the effects of tobacco exposure on these mice has not been established.
Often with a molecular mass over 500 kDa, mucins are 50每80% O-linked oligosaccharides by weight [65]. Mucins may be divided into two major groups, (i) membrane bound and (ii) secreted. Secreted mucins are classified as gel-forming and non-gel forming types. The secretion of mucins occurs through both the constitutive and regulated secretory pathways [66]. As part of the constitutive pathway, mucin-containing vesicles from the trans-Golgi network are released extracellularly, creating a ※baseline activity§ of mucin secretion that is Ca2+-independent. [67]. Tethered mucins are typically trafficked to the cell surface by a constitutive pathway [68, 69]. In the regulated pathway, mucin-containing vesicles are transported from donor to storage compartments. For example, after synthesis in the endoplasmic reticulum, vesicles carrying mucins are transferred to the cis-Golgi for core glycosylation. They then undergo a maturation process in the Golgi and pass through the trans-Golgi to storage granules [66]. The mucin containing granules are trafficked towards the apical plasma membrane for secretion in a Rab GTPase-dependent fashion [66]. Studies with the SPOC1 rat goblet cell line demonstrated that increases in intracellular Ca2+ elicited by purinergic receptor stimulation triggered mucin secretion [70]. Further studies with the protein kinase C activator Phorbol 12-myristate 13-acetate and the Ca2+ ionophore ionomycin in goblet cell mucin secretion demonstrated the involvement of Ca2+ and protein kinase C in this process [71].
Upon secretion, the data of Verdugo et al. demonstrated that mucins ※exploded§ out of vesicles like a ※jack in the box§. The mechanism for this phenomenon reflects the fact that a pore is formed between the secretory granule and the plasma membrane, extracellular Na+ exchanges for Ca2+ in the granule, and mucins are osmotically expelled from the granule onto airway surfaces. A key aspect of this mechanism is that mucins contain negative charges that would normally repel other mucins when they are densely packed. However, the Ca2+ and H+ in the granules shield mucin negative charges and allow the mucins to remain closely packed. This exchange of Ca2+ and H+ for Na+, causes mucins to expand ~600 times when they are secreted [72]. Consequently, mucins are secreted &dry* with water being supplied by CFTR-mediated Cl− secretion via the ciliated cells [73].Ciliated cells of the conducting airways
The motive force for mucociliary clearance in the airways is provided by the ciliated cells of the respiratory tract. There are typically ~200 cilia per cell and their beating at ~10每15 Hz propels mucus and trapped pathogens/particles towards the oropharynx where it is expelled or swallowed in order to maintain pulmonary sterility [74]. Ciliary epithelia are polarized, with cilia being exclusively located at the apical plasma membrane. Ciliary orientation is determined by planar cell polarity proteins and their associated signaling pathways and is initiated before ciliogenesis[See [75] for a comprehensive review]. In airway epithelia, ciliogenesis is under the control of the forkhead box J1 (FoxJ1) transcription factor [76] and knockout of this gene in a mouse model prevents ciliogenesis [77]. More than 600 proteins have been identified in cilia [78]. Motile cilia typically exhibit the ※9+2§ arrangement where two singlet microtubules are surrounded by nine doublet microtubules along with several other components such as dynein arms, radial spokes and other proteins that are required for proper ciliary assembly and beating [79].
Ciliary beating is typically under the control of second messengers similar to those that increase mucin secretion, and elevations in Ca2+, cAMP and cGMP have been shown to increase beat frequency [80每84] [85每88]. This increase may be triggered by Gs-linked GPCRs such as P2Y2-R and adenosine receptors [89]. cAMP/PKA-dependent regulation of ciliary beating is best understood and PKA and A-kinase anchoring proteins (AKAPs) have been found in cilia [90]. In contrast, while Ca2+ is thought to directly increase ciliary beating, in a PKC-independent fashion, PKC activation is thought to slow ciliary beating [91].
Go to:
Role of ion channels in airway mucus hydration
As noted above, in addition to ciliary beating, a major element that must be maintained for efficient mucociliarly clearance is the hydration status of the mucus [12]. The predominant experimental evidence has identified two airway epithelial major ion transport pathways which play crucial roles in determining hydration of airway surfaces, namely anion secretion and cation absorption. These opposing forces are balanced by the surface epithelium to osmotically regulate airway hydration [92]. Na+ absorption across the apical plasma membrane is mediated by the epithelial Na+ channel (ENaC) [93, 94]. Cl− secretion can occur either by CFTR, or by the Ca2+ activated Cl− channel (CaCC, imcluding Anoctamin 1/TMEM16A) [95, 96]. Ciliated cells are known to co-express Anoctamin 1, CFTR and ENaC [97], suggesting that they can both hydrate the ASL and move mucus to maintain lung sterility.
CFTR is an anion channel and adenine nucleotide binding cassette (ABC) protein, belonging to the ABC transporter subfamily [98]. CFTR is unique amongst ABC proteins in two ways. First, although CFTR has structural similarity to ABC proteins and has 12 transmembrane-spanning domains and two nucleotide-binding domains, it does not act as a transporter protein, but rather functions as an anion channel [99每101]. Second, CFTR has an extra intracellular segment known as the regulatory domain or R-domain, which is not a feature of other ABC proteins. The R-domain can be extensively phosphorylated which serves to gate the channel [100, 102]. Typically, ABC proteins bind ATP and use the energy to drive the transport of various molecules across cell membranes [103]. In CFTR, the interactions of ATP with nucleotide binding domains control opening and closing of the channel pore rather than driving solute transport [98]. Disease-causing CFTR mutations have been extensively studied [7]. For example, the F508 CFTR mutation, where phenylalanine at position 508 is deleted, induces CFTR retention in the endoplasmic reticulum, decreases CFTR open probability, and reduces residence time in the plasma membrane.
ENaC is a heterotrimer consisting of 汐, 汕 and 污-subunits [104, 105]. While this is the subunit combination that is typically found in the lung, in other tissues, ENaC may be substituted by a 汛-subunit [84每86]. The 汐- and 污-subunits must be proteolytically cleaved for ENaC to be activated and to conduct Na+ [106, 107]. The function of the 汕-subunit is likely regulatory in nature [87]. 汐 and 污 ENaC subunits can also be ubiquitination on their N-termin by the E3 ubiquitin ligase Nedd4.2, which plays a significant role in determining the channel half-life [108]. Interestingy, knockdown of NEDD4.2 (NEDD4L) in mice leads to upregulation of ENaC and the development of significant lung pathology [109].
When CFTR is activated, Cl− and HCO3− are secreted into the airway lumen, with Na+ and H2O following passively through the paracelluar pathway. This coordinated response results in an isotonic increase in ASL height/volume [12, 110每112]. Conversely, when ENaC is activated, Na+ moves from the airway lumen into the blood and Cl−/H2O follow paracellularly in the same direction, decreasing ASL volume [113]. Cl− is the most abundant anion in the ASL (~120 mM) and provides the driving force for the osmotically induced changes in ASL volume. In contrast, HCO3− is present at smaller concentrations (~30 mM) and likely has proportionately has effect on ASL volume [114]. In addition, HCO3− likely plays an important role in buffering ASL pH [72, 115]
Normal airway epithelia can sense ASL volume and adjust ion transport rates accordingly, using several feedback systems including: (i) soluble volume sensors encoded in the ASL, such as short palate lung and nasal epithelial clone 1 (SPLUNC1) ATP, and adenosine to regulate ENaC and CFTR [116, 117], and (ii) ciliary beating to directly sense ASL/mucus hydration and response with control of ATP release rates [118]. These processes have been extensively reviewed elsewhere [83, 89, 119, 120]. The optimal height of the periciliary layer has been determined to be ~7 米m for normal ASL. This height reflects a well hydrated PCL layer, which is optimal for mucus clearance since it provides a low frictional environment that allows cilia to beat and mucus to flow. A decreased PCL height due to CFTR deficiency in CF and COPD airways has been associated with reduced mucus clearance and impaired lung innate defense [15, 49, 121].
Whilst ENaC must be cleaved to be activated, it can then be inhibited via several different routes. ENaC*s intracellular N-termini interact with PIP2 to open the gate of the channel. Purine nucleotides, such as ATP, acting through Gq-linked P2Y2 receptors, are able to deplete the intracellular face of the plasma membrane of PIP2, which leads to a decrease in ENaC*s open probability[122每124]. ENaC*s open probability can also be affected by cAMP/PKA-induced phosphorylation, which in the airways can be mediated by 2 adrenergic receptors or A2BR purinergic receptors, both of which are Gs-linked and raise cAMP [95]. The number of ENaC subunits at the plasma membrane is also regulated by short palate lung and nasal epithelial clone 1 (SPLUNC1), a 25 kDa protein that is secreted into the ASL by the underlying epithelia. SPLUNC1 binds directly to 汕-ENaC [125], which causes ENaC to be internalized [126]. An 18 amino acid long region of the protein, termed the S18 region, has also been shown to inhibit ENaC and to slow ASL volume absorption [125]. SPLUNC1-dependent regulation of ASL volume is defective in CF airways [127]. That is, CF ASL is mildly acidic due to the lack of HCO3− secretion through CFTR (~pH 7.0 in normal ASL; ~pH 6.5 in CF ASL)[128] and SPLUNC1 is a pH-sensitive protein that fails to regulate ENaC at ≒ pH 6.5 [127, 129]. SPLUNC1 expression may be increased in COPD airways [130]. However, the possible effect of CFTR diminution on ASL pH and subsequent failure of SPLUNC1 to regulate ENaC remains to be tested.
The interactions between CFTR and ENaC are complicated and not fully understood [111, 131, 132]. However, electrophysiological studies have revealed that ENaC is inhibited by cAMP/PKA in the presence of CFTR and activated by cAMP/PKC in CFTR*s absence [133, 134]. Further support of this regulatory interaction emerged from studies with Madin Darby Canine Kidney (MDCK) epithelial cells where recombinant ENaC activity was decreased by coexpression with full-length CFTR [135]. Similar findings emanated from a study of freshly prepared lung slices from wild-type vs. CFTR(−/−) mice where basal open probability of ENaC increased fourfold in absence of CFTR [136]. Despite these studies, how CFTR actually regulates ENaC remains to be determined [111].
It is also not known whether decreased CFTR expression in COPD airways leads to ENaC hyperactivity and emerging in vivo data suggest that ENaC activity may be intrinsically normal in COPD [15, 137]. Crucially, despite reduced levels of CFTR in COPD airways, the remaining CFTR is wild-type and not a disease-causing mutant, and it may be that the residual wild type CFTR activity in COPD airways (~30% of normal activity) is sufficient to prevent ENaC hyperactivity. The situation may change with severe COPD, however, since ENaC is cleaved and activated by many proteases, including neutrophil elastase and cathepsins [138每142]. Indeed, chronic neutrophilia is seen in severe COPD, which may promote cleavage and activation of ENaC due to increased neutrophil elastase levels [143].
Recent data indicate the regulation of ENaC may be abnormal in COPD. Recent data have indicated that extracellular ATP levels are reduced in COPD secretion, in part due to increased ecto-ATPase activities (Anderson, et al, Blue J. in press). Thus, ATP inhibition of ENaC may be reduced in COPD.
Go to:
The effect of tobacco smoke on ciliary beating
Pyrolysed tobacco contains >5000 different chemicals including aldehydes, which are the product of combusted plant material (e.g. cellulose), heavy metals, CO and free radicals [144每146]. Since tobacco smoke is highly oxidized, it is very labile and its composition changes with time after its production [147]. Of the chemicals contained in tobacco smoke, acrolein, acetaldehyde, formaldehyde and Cd2+ are thought to have the biggest effect on the development of pulmonary disease [148]. Tobacco smoke components such as acrolein and acetaldehyde impair ciliary clearance [149]. Ciliary beat frequency has been shown to be significantly decreased in ciliated cells from nasal brushings of moderate and severe COPD patients compared to control and other at-risk subjects [150] and ciliary beat frequency was found to be lower in smoke-exposed subjects [151]. Volatile compounds in cigarette smoke extract are predicted to reduce ciliary beat frequency through a protein kinase C dependent mechanism [152]. Since these measurements were made under flooded conditions (i.e. the thin film ASL was washed away), this effect was likely due to changes in ultrastructure of the cilia rather than to changes in mucus viscosity.
Cigarette smoke (CS) exposure has been shown to impair ciliogenesis and to cause ciliary shortening [153]. Histone deacetylase 6, a ubiquitin ligase that also affects protein acetylation, has recently been implicated in ciliary shortening after CS exposure [154]. Specifically, CS is thought to upregulate histone deacetylase 6*s ubiquitinase activity and promote removal of ciliary proteins, leading to ciliary shortening. Indeed, cells with reduced histone deacetylase 6 expression are resistant to CS-induced ciliary shortening.
Axonemal abnormalities, including changes in the microtubule*s ※9+2§ arrangement, were also found to be about three fold greater in smokers and ex-smokers as compared to nonsmokers [155]. Abnormalities in radial spokes, dynein arms along with nexin links and fused cilia were further observed in smokers [155每159]. Subsequent analyses revealed that hydrogen cyanide, formaldehyde, acrolein, acetaldehyde, ammonia, nitrogen dioxide and phenol all reduced ciliary beating in a similar fashion as cigarette smoke [160].﹛
The effect of tobacco smoke on CFTR-mediated ion transport
Following both acute and chronic cigarette smoke exposure, CFTR mediated Cl− secretion was found to be significantly reduced in vitro and in vivo [15, 137, 161]. This inhibition was both rapid in onset and sustained over extended periods of time. Several mechanisms have been proposed to account for the reduction in CFTR activity:
Effects on CFTR trafficking: After tobacco smoke exposure, CFTR has been shown to rapidly internalize [15, 162] (Figure 1). This internalization is Ca2+ dependent, with the Ca2+ emanating from lysosomes rather than from the endoplasmic reticulum or mitochondria [163]. Recent data has implicated activation of the MEK-Erk1/2-MAPK pathway in this response [162]. Tobacco exposure also caused CFTR aggregation and induced an alteration in solubility of CFTR, which we speculated caused CFTR to accumulate in a perinuclear aggresome-like compartment rather than traffic to the lysosomes or the proteasome [15]. Such changes in CFTR solubility have previously been observed with misfolded CFTR [164每166]. Because there is a peripheral quality control system for surface CFTR [167, 168], it is possible that tobacco exposure induces an acute mis-folding of surface CFTR, leading to rapid internalization.
Direct effects of tobacco smoke metabolites on CFTR function: Acrolein is a highly reactive tobacco smoke metabolite that is known to form covalent adducts with DNA [169, 170] and with proteins [171]. In single channel patch clamp recordings, acrolein has been demonstrated to directly affect CFTR gating [172]. Cigarette smoke is acidic, and 50 standard cigarettes drawn through 50 mls of Frog Ringer solution acidified the solution by about 2 pH units [173]. This cigarette-exposed acidic media inhibited the activity of CFTR expressed in Xenopus laevis oocytes [125]. As the HCO3− secretion is also mediated by CFTR, tobacco smoke exposure also exhibits reduced secretion of HCO3− presumably resulting in further acidification of ASL [174, 175]. To date, however, there no evidence that tobacco exposure actually causes an acidification of the ASL in vivo.
Effects of tobacco smoke and oxidative stress on CFTR expression: Tobacco smoke is highly oxidizing (5℅1014 radicals per puff [176]). Oxidative stress is a well-known inhibitor of CFTR gene expression [177]. Indeed, Cd2+ and other oxidants present in tobacco smoke have been shown to decrease CFTR gene expression and to affect microRNAs, such as Mir-101 and Mir-144, that themselves influence CFTR expression [178]. Cd2+ was also shown to accumulate in the lungs of GOLD4 COPD patients and is negatively correlated with CFTR expression, suggesting that accumulated heavy metals in lung epithelia may act as toxins that continuously inhibit CFTR expression and drive mucus dehydration in severe COPD patients [178, 179].
Emerging data from multiple laboratories have indicated that tobacco exposure leads to airway dehydration in human bronchial epithelial cells cultured at an air-liquid interface (Figure 1) [15, 180]. A single bout of tobacco smoke exposure (i.e. the smoke from one cigarette) rapidly diminished ASL height (within 30 minutes of initiation of exposure), and it took 3每4 hours for ASL height to return to pre-exposure levels [15]. When cultures were exposed to smoke chronically, resting ASL height decreased permanently, indicating a more severe effect of tobacco exposure on ASL homeostasis [179]. The decrease in ASL height was due to changes in active ion transport, as opposed to a simple drying of the ASL by the smoke exposure, as evidenced by studies showing that [181, 182]. pre-inhibition of ENaC prevented the ASL depletion caused by tobacco smoke., Furthermore, the addition of ENaC antagonists after tobacco-smoke induced ASL dehydration resulted in a quicker restoration of ASL height to normal levels, indicating that continued ENaC activity in the absence of CFTR function contributed to the ASL depletion [181].
Go to:
The effect of tobacco smoke on mucus secretion/mucus clearance
Nasal Mucociliary clearance (MCC) has been assessed in vivo with the saccharine transit test. Thus, this test involves placing saccharine in the inferior nasal turbinate and measuring the time taken for the saccharine to be tasted, which is directly proportional to the mean transit of saccharin through the nasal cavity. For example, the longer it takes to taste the saccharine, the slower the MCC rate [183]. Nasal MCC was significantly reduced in smokers compare to nonsmokers as measured using this method [184].
Mucus adhesion contributes to the pathophysiology of chronic infectious airways diseases in different ways. The notion that mucus adhesion imparts airflow obstruction emerged from micro-CT studies. Specifically, Hogg et al, found that the survival rate of COPD patients was dominated by events internal to the airway basement membrane, i.e., epithelial hyperplasia and mucus obstruction [6, 36]. It has also been reported that mucus plugs from COPD lungs are ~7% solids [15, 185]. Our experimental data and theoretical analyses indicate that when mucus dehydration (as indexed by % solids) exceeds 6% solids, mucus clearance slows [48, 55, 186]. Indeed, the biophysical properties of 6.5% mucus became markedly abnormal (2 log differences) as compared to normal 2% mucus [52, 186]. These data predict that more concentrated mucus will be transported by cilia less effectively. In theory, when mucus concentrations exceed 6%, the osmotic pressure exerted by the mucins in the mucus layer will exceed that of the PCL, leading to a collapse of the PCL and adhesion of mucus to airway surfaces [55]. Mucus from CF subjects exhibited similar properties with elevated % solids content and subsequent mucus stasis [35, 187, 188], suggesting that mucus dehydration plays a role in the pathogenesis of both diseases. The ASL dehydration induced by tobacco-smoke mediated down-regulation of CFTR is further exacerbated by the concomitant increase in mucin secretion [189每191]. For example, acrolein increased MUC5AC expression, which contributes to an imbalance between mucin production and salt/water secretion [192, 193].
Go to:
Animal models of COPD
The in vivo and in vitro human data are complemented by data from animal models of COPD. Historically, mice, rats and ferrets have been chronically exposed to tobacco smoke [194每197]. For example, chronic tobacco exposure in mice causes emphysema, which has been attributed to macrophage elastase (MME) production. Six months of cigarette smoke exposure caused macrophage accumulation in murine lungs along with an enlargement of alveolar airspaces [198]. MME (−/−) mice were protected against this response, indicating that protease production is required for alveolar damage [198]. Interestingly, while mice develop the emphysema phenotype, they do not typically exhibit mucus obstruction/CB. One reason for this observation may be that reduced CFTR activity is critical for mucus dehydration in humans, CFTR is little expressed in murine lungs. Consistent with this notion, CFTR knockout mice do not exhibit a significant pulmonary phenotype [199, 200]. The mucociliary clearance rates observed in rodents is also slower than reported in humans, which may further contribute to the species differences in response to cigarette smoke [201, 202]. Currently, and to the best of our knowledge, there are no good rodent spontaneous or environmentally produced models of CB dehydration.Genetically, mucus obstruction has been induced in mice by transgenically overexpressing the 汕-subunit of ENaC, which produced a phenotype of airway surface liquid (ASL) volume depletion that was associated with mucus adhesion/plaque formation and the inability to clear inhaled bacteria that phenocopies CF and CB [203]. Importantly, these animals exhibited a spontaneous mortality of ~60% after 30 days that reflected mucus obstruction [203]. This model has helped establish the relative importance of hydration on airway health. In contrast, a series of mouse models with either no cilia or dysfunctional cilia surprisingly exhibited little or no pulmonary disease phenotype [202]. Diseases that affect other aspects of the innate defense mechanisms of the lung, e.g., alveolar macrophage dysfunction, neutrophil dysfunction, dysfunction of immunoglobulins (both secretory, IgA, and IgG) tend to produce disease of the alveoli rather than airways. Thus, we believe that it is a reasonable conclusion that mechanical (mucus) clearance is the dominant form of innate defense of the airways, and failure to provide this activity produces obstruction and the propensity for chronic infection.
Go to:
Treatment for CFTR diminution and mucus dehydration in COPD
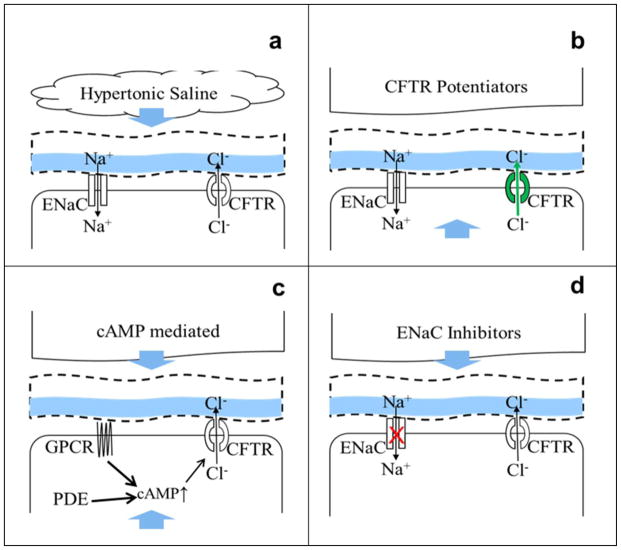
The increased awareness that CB is at least, in part, a disease of CFTR dysfunction and mucus dehydration, suggested that ASL/mucus rehydration would be beneficial in clearing airway mucus obstruction in COPD subjects. This goal may be achieved either by direct rehydration of the mucus with osmotic agents such as hypertonic saline or mannitol, by restoring CFTR function, or by inhibiting ENaC (Figure 2). The use of hypertonic saline for the treatment of dehydrated airways in CF patients has shown promise by improving mucus clearance and lung function, as well as decreasing the number of acute exacerbations (Figure 2a) [204, 205]. Hypertonic saline has also been shown to accelerate MCC in COPD patients [15, 206]. In addition to increasing airway hydration, inhaled hypertonic saline treatment also decreased the number of neutrophils in the lung, suggesting that it may be a viable therapy for COPD patients [207].
﹛
Figure 2
Strategies for treating airway dehydration in CB/COPD
Schema showing a typical airway epithelial cells and different therapies for ASL rehydration. Therapies may be inhaled or taken orally, as indicated by blue arrows. a, Inhaled hypertonic saline directly rehydrates the airway surface, which helps facilitate mucociliary clearance. b, CFTR corrector or potentiator drugs enhances CFTR channel activity and can be taken orally. c, cAMP mediated CFTR enhancement is achieved either through phosphodiesterase (PDE) inhibition or 汕2-adrenergic receptor (GPCR) activation. d, Inhaled ENaC inhibitors reduce Na+ absorption and would enhance the driving force for CFTR-mediated Cl− secretion.
Interestingly, the duration of action of hypertonic saline is relatively short in normal and COPD airways as compared to CF airways [15, 204]. This short response likely reflects the fact that in normal airways, the high salt concentration achieved on airway surfaces following aerosol delivery of hypertonic saline generates chemical gradients for Cl− to be absorbed through CFTR and the paracellular pathway, with Na+ being absorbed through ENaC and also the paracellular pathway, thus limiting the sustainablilty of osmotic effects of hypertonic saline on airway surfaces. In contrast, the absence of CFTR function in CF airways eliminates the transcellular path for Cl− absorption, resulting in the maintenance of high concentrations of NaCl on airway surfaces. Because the high ASL Cl− concentration caused cannot be dissipated as rapidly, a greater, osmotically-induced hydration is generated [135].
The open probability of wild-type CFTR typically ranges from 0.35 to 0.40 [208, 209], and Po is reduced following acrolein exposure to <0.1 [172]. The G551D disease-causing CFTR mutation exhibits an open probability that is ~0 [210]. However, potentiator drugs such as VX770 (Ivacaftor/Kalydeco) have been developed which increase the open probability of both wild-type and G551D CFTR [211]. It has been hypothesized that such potentiators may be a suitable treatment to increase open probability, and hence restore Cl− secretion, in CFTR-deficient COPD airways (Figure 2b). Indeed, acute VX770 addition increases CFTR-mediated Cl− secretion after cigarette smoke extract exposure in HBECs [180]. In contrast, while the F508 CFTR mutant is retained in the endoplasmic reticulum, it may be induced in vitro to traffic to the plasma membrane with a reduced open probability of ~0.1 [212]. CFTR correctors have also been developed that help traffic CFTR to the plasma membrane, although their effects on CS-exposed HBECs have not been determined [213]. As a caveat, it has recently been shown that chronic VX770 exposure may inhibit wild type CFTR by reducing CFTR*s dwell time in the plasma membrane, suggesting that VX770 may not be the optimal potentiator to restore CFTR function in COPD patients [214]. Thus, potentiator/corrector based approaches to restore CFTR function are relatively new and the continued development of compounds designed to improve trafficking of CFTR in tobacco-exposed/CB airways may yield novel therapeutics for the treatment of CFTR dysfunction in CB airways.
There are more conventional approaches to activating CFTR in COPD subjects. Since CFTR is a cAMP-activated channel, phosphodiesterases such as roflumilast may also serve to increase CFTR activation levels (Figure 2c) [182, 215]. This effect may also be present in COPD airways, given that ~30% of CFTR function remains [15, 137, 161]. Similarly, inhaled 2 adrenergic receptor agonists are a mainstay of COPD treatment, and they are thought to relieve the airway obstruction by inducing smooth muscle relaxation, as seen in asthmatic patients [216]. However, these agonists also activate CFTR [217]. To date, no COPD study has been completed in order to determine the most effective 2 agonist for activating CFTR. Such optimization of existing/FDA approved 2 agonists may provide a useful tool for reducing mucus dehydration in CB airways.
A fourth therapeutic approach for COPD focuses on inhibiting ENaC to restore airway hydration (Figure 2d). To date, no ENaC inhibitors have been used to successfully treat airway dehydration by limiting Na+ absorption through the airways and previous trials have failed due to both safety issues and lack of efficacy [218] (amilioride trials- safe- no effect). Amiloride and its analogues are a series of ENaC antagonists that were initially designed to inhibit ENaC in the kidneys with a goal of treating hypertension [219]. Whilst efficacious at increasing MCC, amilioride failed therapeutically to treat CF because it was rapidly absorbed across the airways via organic cation transporters into the systemic circulation where they can gain exposure to ENaC in the kidneys [220]. Inhibition of ENaC in the distal convoluted tubules of the kidneys directly blocks Na+ absorption and indirectly blocks K+ secretion, leading to a diuresis and natriuresis that is ※potassium sparing§ [219]. Consequently, if ENaC inhibitors delivered to the lung via aerosol are absorbed systemically and cleared renally, hyperkalemia can result. Two inhaled ENaC antagonists (amiloride and GS9411) have elicited a renal response [218, 221]. Recently, novel ENaC antagonists have been administered by inhalation and shown to increase rehydration and ciliary beating without eliciting this response, suggesting that ENaC antagonism may be a viable therapy for ASL rehydration in COPD airways [222, 223]. Furthermore, ENaC antagonists have been shown to reverse ASL dehydration seen in tobacco-exposed airway epithelia, and to increase ciliary beat frequency in vitro, suggesting that if safety issues can be overcome, then ENaC antagonists may be a useful treatment for CB.Using components of the MCC pathway as biomarkers of exposure for the study of new and emerging tobacco products
In addition to the existing brands of tobacco that have been well characterized (e.g. Marlboro, Camel cigarettes etc.), a large number ※new and emerging§ tobacco products and e-cigarettes are reaching the market that are currently unregulated by the US Food and Drug Administration. These products include little cigars, E-cigarettes and hookah [224]. For these new and emerging tobacco products, their effects on the lung are only beginning to be studied. It has been proposed that E-cigarettes, which produce a vapor with an electronic coil, may lessen the risk of developing COPD and/or lung cancer because they do not combust tobacco nor require pyrolysis to produce the vapor [225, 226]. Whilst the principle ingredients of E-cigarettes are typically propylene glycol, nicotine and flavorings, the chronic effect of these devices on the lungs is only beginning to be understood, and their propensity for triggering COPD is not known. For example, it has recently been reported that E-cigarette liquids promoted IL-6 production and inhibited SPLUNC1 expression [227]. IL-6 is known to increase mucin gene expression and secretion [228], while SPLUNC1 not only regulates ENaC (see above, but also plays multiple other roles in innate defense) and its absence could lead to altered epithelial function. Furthermore, formaldehyde has recently been detected in e-cigarette vapor, and this agent may be a breakdown product from the propylene glycol propellant [229]. As these tobacco products are newly emerging, appropriate human studies will need to be performed before these experiments are fully validated and their long term effects understood.
Chemical flavors are now widely being used in new and emerging tobacco products [230每232]. However, while these flavors are generally safe to eat, by and large, their effects on the lung are unknown. Recent data have indicated that flavors such as menthol can stimulate transient potential receptor (TRP) channels in the airways. TRP channels are involved in nociception in the airways and their stimulation reduces irritation caused by tobacco exposure, making menthol cigarettes easier to smoke than regular cigarettes [233]. Indeed, genetic variants in TRPA1 amongst the general population have been linked to an increased preference for menthol cigarettes and for heavier tobacco intake in a subset of the population [234]. Another flavor that has received attention is diacetyl, which is used in the food industry to provide a buttery flavor and is added to many food products, including popcorn and beer [235]. Diacetyl is thought to be safe when ingested orally and is approved for human usage [235]. However, diacetyl inhalation is known to cause bronchiolitis obliterans or ※popcorn workers lung§ [236]. Additionally, the authors reported a twofold greater risk of diacetyl causing COPD, although these cases may have been bronchiolitis obliterans that were misdiagnosed. Thus, it may wise that every flavor used in new and emerging tobacco products and e-cigarettes be retested for lung toxicity in order to prevent the development of chronic airway disease.
Considering the present shift in tobacco use from conventional cigarettes to new and emerging tobacco products like little cigars, smokeless tobacco and e-cigarettes, the effects that these new products will exert on airway hydration and associated mucociliary clearance are as yet untested [237每239]. Importantly, components of the mucociliary transport system will serve as useful biomarkers of exposure both in vivo and in vitro. In vivo, CFTR activity/expression, mucus clearance rates, induced sputum properties (mucus hydration, protease activity) are all useful and relatively accessible biomarkers that can be used to measure effects of exposure. Similarly, CFTR function, ciliogenesis, mucin expression and mucus clearance rates can all be assayed in well-differentiated HBECs in vitro following tobacco/e-cig exposure, suggesting that these measures will be important biomarkers that can be utilized to assess the relative toxicity of exposure to new and emerging tobacco products.
The differences between chronic bronchitis and cystic fibrosis
Although we speculate that CF and CB are characterized by relative dehydration of mucus and mucus adhesion, the pathophysiologic sequences that produce this state are very different in the two diseases. We hypothesize that the pathogenesis of CF lung disease directly reflects abnormal regulation of electrolyte transport to produce ASL volume depletion. Specifically, we speculate that the failure of CFTR function in CF airways produces an intrinsic defect in regulation of Na+ absorption and a failure to secrete anions via the tonic basal anion secretory pathway, i.e., CFTR itself. Concurrently, reduced HCO3− secretion due to the lack of CFTR contributes to a mildly acidic pH [127]. Thus, the primary defect is one of ASL volume depletion, and only later, when chronic infection sets in, does mucin hypersecretion commence, which worsens the relative dehydration state of mucus.
In contrast, mucus dehydration in CB is likely multifactorial. Mucin hypersecretion has been thought to be the primary defect in COPD that leads to relative airway surface dehydration [74]. Indeed, it is highly likely that high rates of secretion of mucins into the airway lumen, coupled with upregulated ecto-ATPase mediated reductions in extracellular ATP concentrations, contribute to increased mucus percent solids content. However, new data suggests that CFTR dysfunction contributes equally to this phenotype [11] [Anderson, Blue J 2015). Compared to CF, COPD is a relatively slowly evolving disease when left untreated. Data from CF subjects suggest that severe CF mutations lead to a relatively complete inhibition of CFTR function and relatively rapid mucus plugging and heterogeneous loss of mucus clearance. In contrast, since tobacco exposure results in a progressive decline in CFTR function (50% in smokers, >70% in COPD patients), mucus dehydration is not so severe (~10% solids in COPD; up to 20% solids in CF) so the mucus obstruction is not so profound, consistent with a slower onset of disease.
Both diseases exhibit chronic inflammation. Inflammation in CF is characterized by high intraluminal IL 8 levels and high concentrations of polymorphonuclear cells [240]. The inflammatory response of the airway in COPD patients is likely more complex [241]. Perhaps this complexity reflects the chronic exposure to a tobacco smoke mixture that contains more than 5,000 chemical entities. Often, early in the disease, the inflammatory response is characterized by increased intraluminal and airway wall mononuclear cells, particularly CD4 and CD8 cells [241]. Only later in the disease, once severe obstruction of the airways obstruction and chronic infection sets in, does persistent neutrophilic accumulation in mucus become characteristic of this disease process [143]. Despite these differences, our knowledge of CF has helped drive a better understanding of the CFTR and mucus defect in COPD.Airway Hydration and COPD
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4567929/#R15﹛