肝巨噬细胞Kupffer细胞在肝病发病机制中的作用
Role of liver macrophages Kupffer cells in the pathogenesis of liver disease
Kupffer细胞,常驻肝巨噬细胞长期以来被认为是主要清除细胞,负责从门脉循环中去除颗粒物质。然而,主要来自动物模型的证据表明Kupffer细胞可能涉及各种肝病的发病机理,包括病毒性肝炎,脂肪性肝炎,酒精性肝病,肝内胆汁淤积,肝移植期间肝脏的激活或排斥以及肝纤维化。本文综述了越来越多的证据,表明Kupffer细胞可能通过在肝脏病毒感染期间产生有害的可溶性介质以及抗原呈递细胞而在肝细胞的破坏中起到效应细胞的作用。此外,它们可能代表细胞毒性CD8和调节性T细胞的化学引诱物分子的重要来源。它们在纤维化中的作用已经确立,因为它们是TGFβ1产生的主要来源之一,其导致星状细胞转化为肌成纤维细胞。肝脏中的所有这些可变功能是否由不同的Kupffer细胞亚群介导仍有待评估。在这篇综述中,我们提出了一个模型,证明了库普弗细胞在肝病发病机制中的作用。
关键词:Kupffer细胞,肝病,肝损伤,肝纤维化,肝细胞癌,肝炎,脂肪性肝炎。
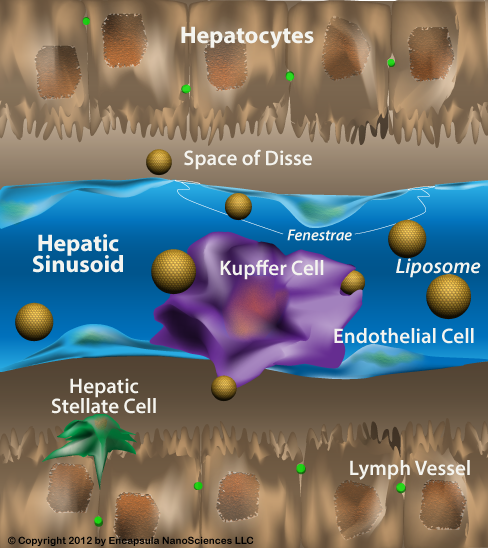
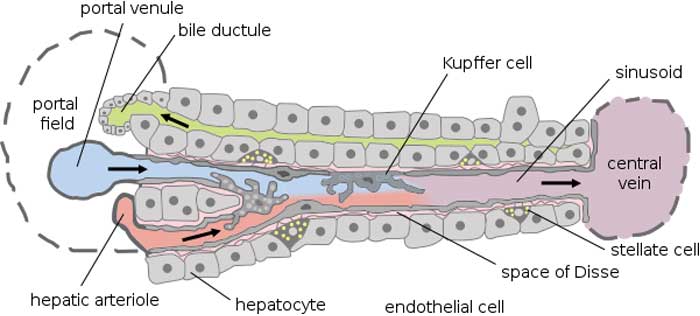
肝脏的肝血窦内衬包含非实质细胞群,其由Kupffer细胞(KCs),窦内皮细胞(SEC)和星状细胞(SC)组成。所有这三种细胞类型似乎在肝脏稳态和肝脏疾病的发病机制中起着至关重要的作用[1]。 KCs构成网状内皮系统中组织巨噬细胞的80%-90%,占肝细胞总数的约15%[2]。它们主要存在于小叶的门静脉区域(43%),但KCs也存在于中区(28%)和中央区(29%)[2]。尽管认为KCs是固定的肝脏组织巨噬细胞,但有证据表明它们具有沿着正弦壁迁移的能力,平均速度为4.6±2.6(SD)微米/分钟[3]。自1876年由von Kupffer描述这些常驻肝脏巨噬细胞以来,已经提出了关于它们的起源和参与肝脏稳态和损伤的各种理论。值得注意的是,几乎所有关于Kupffer细胞作用的证据都来自动物模型。
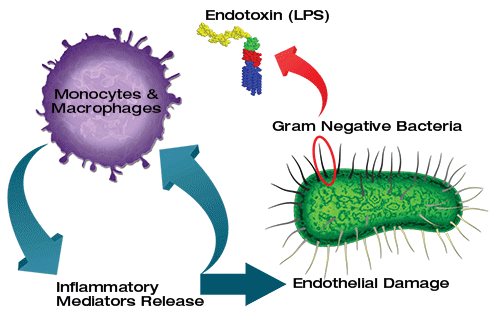
KCs是第一个暴露于从胃肠道吸收的物质的细胞。它们消除和解毒微生物,内毒素,变性细胞,免疫复合物和毒性剂(例如乙醇)的能力是重要的生理功能。由于它们的关键位置,KCs可能作为抗原呈递细胞[4]并参与肿瘤监测[5]和肝脏的再生过程[6]。它们似乎也通过可溶性炎症介质的表达和分泌在先天免疫反应和宿主防御中发挥关键作用[7]。有越来越多的证据表明KC和脂多糖(LPS)之间的相互作用可能是导致各种类型肝损伤肝毒性的起始事件,包括内毒素血症,酒精性肝损伤和缺血/再灌注损伤[8,9]和全身性病毒感染[10]。 ]。
Role of liver macrophages Kupffer cells in the pathogenesis of liver disease
Kupffer cells, the resident liver macrophages have long been considered as mostly scavenger cells responsible for removing particulate material from the portal circulation. However, evidence derived mostly from animal models, indicates that Kupffer cells may be implicated in the pathogenesis of various liver diseases including viral hepatitis, steatohepatitis, alcoholic liver disease, intrahepatic cholestasis, activation or rejection of the liver during liver transplantation and liver fibrosis. There is accumulating evidence, reviewed in this paper, suggesting that Kupffer cells may act both as effector cells in the destruction of hepatocytes by producing harmful soluble mediators as well as antigen presenting cells during viral infections of the liver. Moreover they may represent a significant source of chemoattractant molecules for cytotoxic CD8 and regulatory T cells. Their role in fibrosis is well established as they are one of the main sources of TGFβ1 production, which leads to the transformation of stellate cells into myofibroblasts. Whether all these variable functions in the liver are mediated by different Kupffer cell subpopulations remains to be evaluated. In this review we propose a model that demonstrates the role of Kupffer cells in the pathogenesis of liver disease.
Keywords: Kupffer cells, Liver disease, Hepatic injury, Liver fibrosis, Hepatocellular carcinoma, Hepatitis, Steatohepatitis
The sinusoidal lining of the liver contains the nonparenchymal cell populations which consist of Kupffer cells (KCs), sinusoidal endothelial cells (SEC) and stellate cells (SC). All three cell-types seem to play a crucial role in liver homeostasis and in the pathogenesis of liver disease[1]. KCs constitute 80%-90% of the tissue macrophages in the reticuloendothelial system and account for approximately 15% of the total liver cell population[2]. They are mainly found in the periportal area of the lobule (43%), but KCs also exist in the midzonal (28%) and in the central area (29%)[2]. Despite the view that KCs are fixed tissue macrophages of the liver, there is evidence that they have the ability to migrate along sinusoidal walls with a mean speed of 4.6 ± 2.6 (SD) microns/min[3]. Since the description of these resident liver macrophages in 1876 by von Kupffer various theories have been proposed with regard to their origin and involvement in liver homeostasis and injury. It should be noted that almost all available evidence for the role of Kupffer cells comes from animal models.
KCs are the first cells to be exposed to materials absorbed from the gastrointestinal tract. Their ability to eliminate and detoxify microorganisms, endotoxins, degenerated cells, immune complexes, and toxic agents (e.g. ethanol) is an important physiological function. Due to their key location, KCs might function as antigen-presenting cells[4] and participate in tumour surveillance[5] and the regeneration processes of the liver[6]. They also seem to play a key role in innate immune responses and host defence through the expression and secretion of soluble inflammatory mediators[7]. There is accumulating evidence that the interaction between KC and lipopolysaccharide (LPS) may be the initiating event leading to hepatotoxicity in various types of liver injury including endotoxinaemia, alcoholic liver injury and ischemia/reperfusion injury[8,9] and systemic viral infections[10].
REFERENCES
Role of Kupffer cells in the pathogenesis of liver disease https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4087584/
Liver macrophages: Friend or foe during hepatitis B infection?
Liver Int. 2018 Oct;38(10):1718-1729. doi: 10.1111/liv.13884. Epub 2018 Jun 9.
Faure-Dupuy S1,2, Durantel D1,2,3, Lucifora J1,2. Author information 1INSERM U1052, CNRS UMR-5286, Cancer Research Center of Lyon (CRCL), Lyon, France.2University of Lyon, University Claude-Bernard (UCBL), Lyon, France.3Laboratoire d'excellence (LabEx), DEVweCAN, Lyon, France.
The Hepatitis B virus chronically infects the liver of 250 million people worldwide.
Over the past decades, major advances have been made in the understanding of Hepatitis B virus life cycle in hepatocytes. Beside these parenchymal cells, the liver also contains resident and infiltrating myeloid cells involved in immune responses to pathogens and much less is known about their interplay with Hepatitis B virus.
In this review, we summarized and discussed the current knowledge of the role of liver macrophages (including Kupffer cells and liver monocyte-derived macrophages), in HBV infection. While it is still unclear if liver macrophages play a role in the establishment and persistence of HBV infection, several studies disclosed data suggesting that HBV would favour liver macrophage anti-inflammatory phenotypes and thereby increase liver tolerance.
In addition, alternatively activated liver macrophages might also play in the long term a key role in hepatitis B-associated pathogenesis, especially through the activation of hepatic stellate cells.
Therapies aiming at a transient activation of pro-inflammatory liver macrophages should therefore be considered for the treatment of chronic HBV infection.
KEYWORDS:
Kupffer cells; hepatitis B virus; innate immunity inhibition; tolerance
https://www.ncbi.nlm.nih.gov/pubmed/29772112
Histology, Kupffer Cell
Michael L. Tan; Daniel R. Webster.
INTRODUCTION
Kupffer cells (also known as stellate sinusoidal macrophages or Kupffer-Browicz cells) are macrophages found in the sinusoids of the liver. In fact, Kupffer cells make up 80% to 90% of all the macrophages in the entire human body. They are a component of the host immune system and are involved in the metabolism of various compounds.
FUNCTION
The life-span of a Kupffer cell is estimated to be 3.8 days. The main role of Kupffer cells is to clear foreign debris and particles from the portal system circulation that pass the liver. Kupffer cells can ingest large particles via phagocytosis and small particles and molecules via pinocytosis. It has also been shown that Kupffer cells can migrate to the portal areas and hepatic lymph nodes before they die. In the liver, the population of Kupffer cells is constant, regulated by apoptosis and phagocytized by neighboring Kupffer cells. In contrast to monocyte-derived macrophages with no proliferative potential, Kupffer cells have a proliferative capacity, allowing regeneration of itself. In granuloma formation, Kupffer cells are activated without a supply of monocytes, transforming into multinuclear giant cells.
https://www.ncbi.nlm.nih.gov/books/NBK493226/
Kupffer Cells Hasten Resolution of Liver Immunopathology in Mouse Models of Viral Hepatitis
Giovanni Sitia , Matteo Iannacone , Roberto Aiolfi, Masanori Isogawa, Nico van Rooijen, Cristina Scozzesi, Marco E. Bianchi, Ulrich H. von Andrian, Francis V. Chisari, Luca G. Guidotti
Published: June 2, 2011https://doi.org/10.1371/journal.ppat.1002061
Abstract
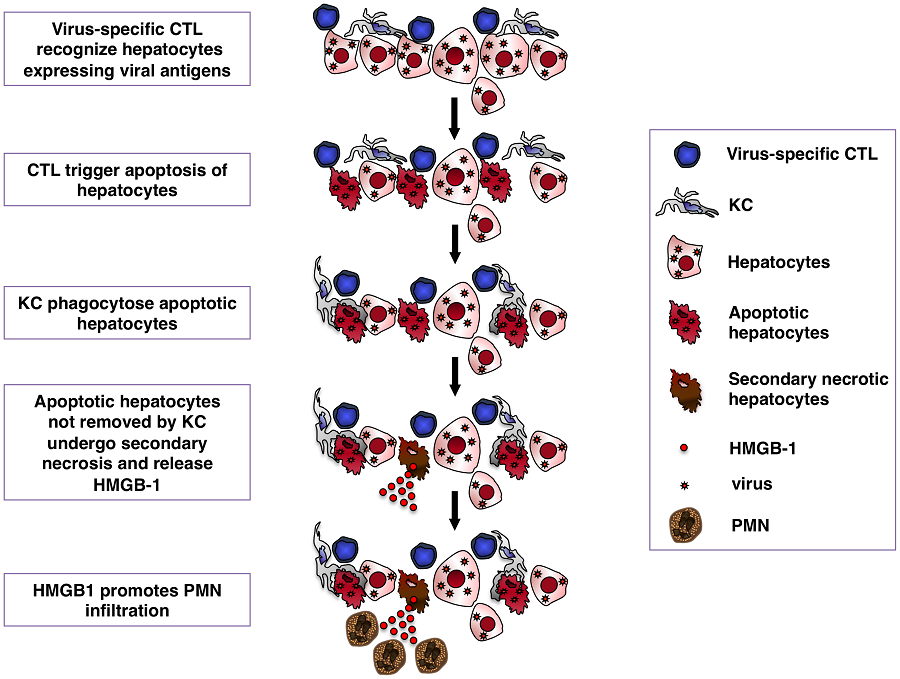
Kupffer cells (KCs) are widely considered important contributors to liver injury during viral hepatitis due to their pro-inflammatory activity. Herein we utilized hepatitis B virus (HBV)-replication competent transgenic mice and wild-type mice infected with a hepatotropic adenovirus to demonstrate that KCs do not directly induce hepatocellular injury nor do they affect the pathogenic potential of virus-specific CD8 T cells. Instead, KCs limit the severity of liver immunopathology. Mechanistically, our results are most compatible with the hypothesis that KCs contain liver immunopathology by removing apoptotic hepatocytes in a manner largely dependent on scavenger receptors. Apoptotic hepatocytes not readily removed by KCs become secondarily necrotic and release high-mobility group box 1 (HMGB-1) protein, promoting organ infiltration by inflammatory cells, particularly neutrophils. Overall, these results indicate that KCs resolve rather than worsen liver immunopathology.
Author Summary
Kupffer cells (KCs), the resident macrophages of the liver, are considered important contributors to liver injury during viral hepatitis due to their pro-inflammatory activity. Herein we utilized two different mouse models of viral hepatitis (where liver damage is triggered, as during viral hepatitis in humans, by virus-specific CD8 T cells) to show that KCs do not directly induce liver injury nor do they affect the pathogenic potential of virus-specific CD8 T cells. Instead, KCs limit the severity of liver immunopathology. Mechanistically, our results are most compatible with the hypothesis that KCs contain liver immunopathology by removing dying hepatocytes. Dying hepatocytes not readily removed by KCs release high-mobility group box 1 (HMGB-1) protein, promoting organ infiltration by inflammatory cells, particularly neutrophils. These results indicate that KCs resolve rather than worsen liver disease.
In one model effector HBV-specific CD8 T cells are adoptively transferred into immunocompetent transgenic mice that replicate HBV at high levels in the hepatocyte [16]–[21]. In a second model wild-type mice previously immunized with a plasmid encoding β-galactosidase (β-Gal) are infected with a β-Gal-expressing hepatocyte-tropic adenovirus [21], [22]. Both models revealed that KCs have no impact on the ability of virus-specific CD8 T cells to home to the liver, recognize antigen and kill hepatocytes, nor do they significantly act as effector cells to destroy the hepatocytes. Instead, KCs reduce the overall severity of T cell-mediated immunopathology by removing apoptotic hepatocytes from the liver.
https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1002061

.png)
.png)